Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353versão On-line ISSN 2346-2183
Dyna rev.fac.nac.minas v.78 n.168 Medellín out./dez. 2011
OPTIMIZATION OF THE BASIC ETHANOLYSIS OF RICIN OIL USING THE RESPONSE SURFACE METHODOLOGY
OPTIMIZACIÓN DE LA ETANÓLISIS BÁSICA DEL ACEITE DE RICINO USANDO LA METODOLOGÍA DE LA SUPERFICIE DE RESPUESTA
Jorge Montoya
Facultad de Minas, Universidad Nacional de Colombia, Sede Medellín. jimontoy@gmail.com
Pedro Benjumea
Facultad de Minas, Universidad Nacional de Colombia, Sede Medellín. pbenjume@unal.edu.co
Veselina Pashova
Facultad de Minas, Universidad Nacional de Colombia, Sede Medellín. vpashova@yahoo.es
Received for review September 22th, 2009, accepted January 15th, 2011, final version February, 15th, 2010
ABSTRACT: Biodiesel production from ricin oil has been the focus of several pieces of research in recent years. However, few studies related to the basic ethanolysis of this non-edible vegetable oil have been reported. In this work, the optimum conditions for maximizing the conversion of the basic ethanolysis of ricin oil were determined using the response surface methodology (RSM). Experiments were conducted using a 2k rotable central composite design. The process variables were evaluated at two levels: alcohol/oil molar ratio (3/1- 10/1), catalyst quantity (1%-1.5%), and temperature (20 °C-35 °C). In order to ensure a good adjustment of the design, a quadratic model was implemented for the response variable. The model was able to predict 99.663% of the total variation of the system. The optimum conversion was of 93.64%, obtained with an alcohol/oil molar ratio of 9.86/1, a catalyst concentration of 1.2% with respect to oil weight, and a reaction temperature of 30 °C, for a period of 1 hour.
KEYWORDS: Ricin oil, ethanolysis, response surface
RESUMEN: La producción de biodiesel de aceite de ricino ha sido el objeto de varias investigaciones en años recientes. Sin embargo, se han reportado pocos estudios relacionados con la etanólisis básica de este aceite vegetal no comestible. En este trabajo se determinaron las condiciones óptimas para maximizar la conversión de la etanólisis básica del aceite de ricino utilizando la metodología de la superficie de respuesta. Los experimentos fueron ejecutados usando un diseño 2k central, compuesto y rotable. Las variables del proceso se evaluaron en dos niveles: relación molar alcohol/aceite (3/1-10/1), cantidad de catalizador (1%- 1.5%), y temperatura (20 °C-35 °C). Para asegurar un buen ajuste del diseño se implementó un modelo cuadrático para la variable respuesta. El modelo logró predecir un 99.663% de la variación total del sistema. La conversión óptima fue de 93.64%, obtenida con una relación molar alcohol/aceite de 9.86/1, una concentración de catalizador de 1.2% con respecto a la masa de aceite, y una temperatura de reacción de 30°C, por un período de 1 hora.
PALABRAS CLAVE: Aceite de ricino, etanólisis, superficie de respuesta
1. INTRODUCTION
The development of alternative sources of energy, other than those obtained from oil, has gained importance in recent years; mainly due to the growing energy demand, the more strict control of gas emissions into the atmosphere, and the scarcity of fossil fuel resources.
Biodiesel derived from animal fats and vegetable oils constitutes an alternative to partially replace fossil fuels. However, in recent years there has been a global alert caused by the fragile food security which has led to the research on new raw materials that do not threaten such food security. A promising alternative for obtaining biodiesel is the use of non-edible oils such as ricin oil. The high viscosity of this oil is an issue that can be solved by chemically converting the triglycerides into alkyl-esters.
Although there are different routes for producing fuels from vegetable oils, transesterification has been the one most commonly applied [1-2]. Temperature, catalyst concentration and nature, the type of alcohol, the alcohol/oil molar ratio, and agitation level have been reported as the main variables affecting the transesterification reaction [3-4].
Even though agitation plays an important role at the beginning of the transesterification process, since the idea is to get the reactants into close contact with each other in such way that the resistance to the mass transfer can be overcome, this factor gradually loses importance as the reactor content decreases its viscosity [5].
Vegetable oil transesterification processes using basic catalysts are particularly faster than those catalyzed under acidic conditions [6]. Due to this reason, along with the fact that the basic catalysts are less corrosive than acidic compounds, homogeneous basic catalysis have been preferred in conventional industrial processes which commonly use alkaline metals, alkoxides, and hydroxides [7]. It is also possible to carry out the transesterification reaction with heterogeneous and enzymatic catalysts, and even without using any catalysts [8-9].
Regarding the alcohol used in the transesterification or alcoholysis reaction, methanol has been the most common option due to its wider availability, lower price, and higher reactivity [10]. However, methanol is mainly produced from natural gas which constitutes a raw material of fossil origin. The use of ethanol (ethanolysis), on the other hand, allows for the production of an entirely renewable biodiesel since this alcohol is mainly produced from biomass.
Other advantages of ethanol over methanol are ethanol's comparatively low toxicity, and the fact that the extra carbon in the molecule slightly increases the heat content and cetane number of the resulting biodiesel [11]. On the other hand, ethanolysis may have more technical problems than methanolysis. It is more energy consuming and some traces of water in the reaction mixture can have dramatic effects on ethyl-esters yield. Only a 30% conversion for the ethanolysis of rapeseed oil with hydrated ethanol (95%), compared to 94-95% under water-free conditions has been reported [11].
The main studies related to the alcoholysis of several conventional vegetable oils as well as ricin oil have focused on determining the effects of the type of alcohol and the nature and concentration of the catalyst on reaction conversion [12-13]. In that sense, some statistical tools have been used. The response surface methodology (RSM) has been successfully used in determining the influence and importance of some variables of interest on the transesterification reaction, as well as in optimizing biodiesel yield. Vicente et al. used the RSM to study the optimization of the methanolysis of sunflower oil with different types of homogenous catalysts [14]. Hass et al., using RSM, studied the in-situ transesterification of soybean oil and reported an optimum methyl-esters conversion of 95%, achieved with the following reaction conditions: temperature of 23°C, methanol/oil/catalyst (alkaline) molar ratio of 543/1/2, during an 8 hr reaction period [15]. Rashid et al. optimized the methanolysis of rapeseed oil with the use of different types of basic catalysts obtaining a maximum conversion of 95%, using 1% (w/w) KOH, a methanol/oil/ molar ratio of 6/1, a temperature of 60 °C, and a mixing speed of 600 rpm, for a period of 2 hrs [16]. Domingos et al. evaluated the ethanolysis of R. sativus oil, determining as optimum reaction conditions the following: ethanol/oil molar ratio of 11.7/1, concentration of NaOH of 0.4% (w/w), a temperature of 45°C, and vigorous agitation for a period of 60 mins [17]. The use of the RSM technique has not been limited to the study of the alkyl-esters conversion. Ghadge et al. used RSM to optimize the reduction of free fatty acids (FFA) in mahua oil to 1% by pre-treating the oil with 0.32% (v/v) of methanol, 1.24% (w/w) of H2SO4, and a temperature of 60°C, for a period of 1.26 hrs [18].
The aim of this work is to use the RSM in order to determine the reaction conditions for which the conversion of ethyl-esters from ricin oil is at its maximum, using NaOH as the catalyst. A mathematical model was devised for determining the effect of variables such as: catalyst concentration, reaction temperature, and alcohol/oil molar ratio. Agitation remained constant at a speed level of 350 rpm.
2. METHODOLOGY
2.1 Materials and methods
Ricin oil was supplied by ANDERCOL S.A. ethylic alcohol with a 99% purity grade (v/v) of Merck reference. Sodium hydroxide was obtained pure and in grains of Carlo Erba reference. The 95% pure ethyl ricinoleate was used in the chromatographic tests. The quantification of species was carried out using the internal standard methodology and using 99% pure enanthate of Carlo Erba reference.
The ricin oil acid index, saponification index, unsaponifiable matter, and iodine number were determined using the ASTM standard methods D-1980, D-5558, D-1965, and D-5554, respectively. The molecular weight of the oil was measured by cryoscopy.
The fatty acid profile of the ricin oil was determined by means of gaseous chromatography. The quantification of the ethyl-esters was carried out according to the ASTM D-6584 standard method, using a Varian 3800 gas chromatograph with an FID detector. The column used in the analysis was a Varian CP 9076, 10m x 0.32mm x 0.1mm, esters and glycerides selective. The chromatograph was programmed to work at two temperature ramps, one starting from 50 °C up to 150 °C, at a rate of 5 °C/min; and the other from 150 °C up to 250 °C, at a heating rate of 7 °C/min.
2.2 Experimental design
Three process variables at two levels were chosen for the design of experiments: R, the alcohol/oil molar ratio (3/1-10/1); C, the catalyst concentration (1%-1.5%); and T, the reaction temperature (20°C-30°C). The levels used for each variable were based on a series of previous trials.
The response variable used to build the design of experiments was the conversion of the reaction in terms of ethyl-esters weight percent after the glycerol separation and drying processes. Experiments were conducted using a 2k (k=3) rotable central composite design. This type of design, entailing 23 experiments (2k=8 main experiments, 2k=6 axial points and n=9 replicates at the center point), is able to explore curvatures and non-lineal behaviors of the response variable. The experimental design and the construction of the response surface were carried out using the software Statgraphics 5.0TM.
2.3 Ethanolysis reaction
The reactions were carried out in a 500 ml three-neck round-bottom reactor placed on a magnetic stirrer hot plate. The reactor was immersed in a thermal oil bath and connected to a reflux condenser system in order to ensure a constant reaction temperature and to avoid alcohol losses through evaporation.
In each experiment, initially, 100 g of ricin oil were weighted and poured in the three-neck round-bottom reactor. Then, a sodium ethoxide alcoholic solution was prepared in a separate beaker, taking the adequate alcohol/oil molar ratio and catalyst concentration into account. Subsequently, the alcoholic solution was added to the ricin oil and the reaction was left to proceed for an hour at the selected temperature and agitation speed.
Once the reaction ended, the next step was to separate the ethyl-esters and glycerol phases and then to wash and dry the biodiesel. For this purpose, the entire content of the biodiesel phase was poured into a separation funnel with an equal volume of water at a temperature of 60 °C. The funnel was continuously stirred to ensure a good biodiesel-water contact. This procedure was repeated until the water came out clear from the funnel. Finally, the washed biodiesel was dried using a heating plate at a temperature close to 100°C in order to evaporate the residual water and ethanol.
3. RESULTS AND DISCUSSION
3.1 Characterization of ricin oil
The fatty acid profile of the ricin oil consisted of 1.30 % (w/w) palmitic acid, 1.55 % (w/w) stearic acid, 3.97 % (w/w) oleic acid, 5.1% (w/w) linoleic acid, 0.53% (w/w) linolenic acid, and 87.55% (w/w) ricinoleic acid.
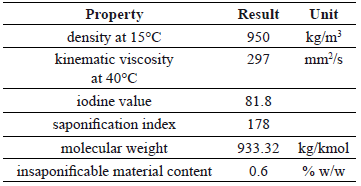
Table 1 shows the main properties of ricin oil. Its molecular weight was very similar to that of the simple triglyceride formed from ricinoleic acid (933 g/mol). This value agrees with the high content of this triglyceride in the parental oil. The higher values of density and viscosity of the ricin oil in comparison with conventional vegetable oils such as palm, soy, and sunflower, can be explained as a consequence of the presence of the hydroxyl group bounded to the triglyceride molecule. The low acid index guarantees a low soap formation during oil transesterification.
Table 1. Properties of ricin oil
3.2 Ethanolysis optimization
The mass recovery of ethyl-esters (% w/w) corresponding to each point of the randomized experimental design is shown in Table 2.
Table 2. Results of the randomized experimental design
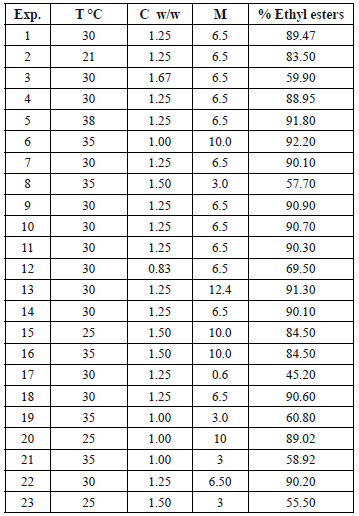
Table 3 shows the statistical data obtained from the analysis of variance (ANOVA table) for each of the variables studied in the experiment.
Table 3. Analysis of variance of the quadratic model developed in the design of experiments
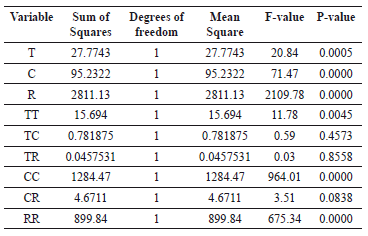
The ANOVA table divides the variability of the response (% w/w of ethyl-esters) into different separate segments corresponding to each factor; and then tests the statistical significance of such factors by comparing their mean square with an estimate of the experimental error. The main effects of these factors on the response variable, along with their magnitudes, are shown in the Pareto chart (Figure 1). The factors that have a significant effect (p-values < 0.05) with a significance level of 9 5% are those that surpass the vertical line. The length of each bar is proportional to the standardized effect which is the relationship between the estimated effect and its standard error.
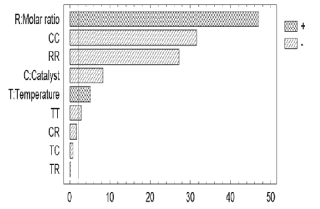
Figure 1. Standardized Pareto chart
As can be seen from the Pareto chart, the three variables under study affected ethyl-esters production. The alcohol/oil molar ratio exhibited the strongest positive effect. The crossed factors (TT, CC, and RR) do not have physical meaning and are used to improve the mathematical adjustment of the statistical model based on the experimental data.
The Pareto chart is not able to explain the magnitude of each effect when a factor goes from a low level to a high one. It is possible that at a low level a factor has a positive effect while at a high level the effect is negative. Figure 2 shows the effect of the variation of the main factors at the different levels. The factor of interest varies from its lowest value to its highest one while the other factors remain constants at its lowest value.
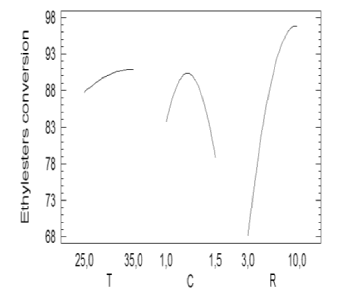
Figure 2. Effect of the variation of the main factors on ethyl-esters yield
From Figure 2 it can be seen that the catalyst concentration followed a parabolic behavior in such way that its maximum point corresponded to the highest ethyl-ester production. Higher values of catalyst concentration can lead to gel and soap formation. The alcohol/oil molar ratio did not show a clear trend, so two additional experiments were carried out for two higher values (15/1 and 20/1). The ethyl-esters percentages obtained for the additional tests (91.12 % and 91.98 %) suggested a flat curve trend for values of R greater than 10/1. Temperature variation subtly affected ethylester yield.
The statistical analysis allows for us to obtain a mathematical model useful for simulating and predicting results within the operation interval for each variable. In this case, the model was adjusted to a second order polynomial equation of the type:
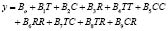 (1)
(1)
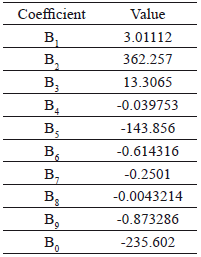
where y corresponds to the response variable (% w/w of ethyl-esters). Table 4 shows the values of the model coefficients.
Table 4. Regression coefficients for the statistical model
The value of the regression coefficient for this model (99.6629) indicates that the quadratic model was able to predict almost 99.7 % of the total variance of the system. The associated mean error was around 1.15 %, and the absolute error of the media was 0.6553 %. The accuracy of the model was checked by comparing the predicted and experimental values for the 23 tests carried out. The experimental and predicted values were adjusted by means of a straight line (Figure 3) with a high level of correlation (regression coefficient = 0.9982).
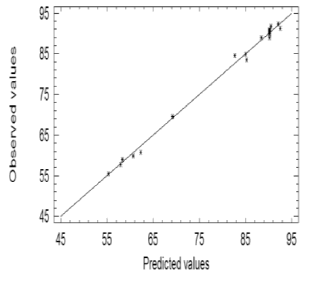
Figure 3. Correlation between predicted and observed values
Based on the high statistical significance of the regression described in (1), the mathematical model was used to generate the response surface (Figure 4) useful for determining the optimum point within the experimental interval.
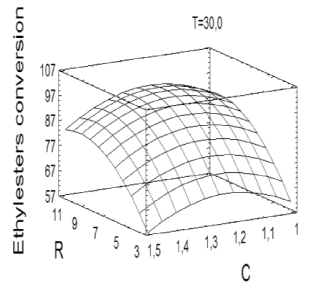
Figure 4. Response surface derived from the optimization of the ethanolysis of ricin oil.
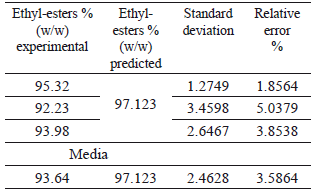
The RSM indicated that the optimal conditions for the ethanolysis of ricin oil corresponded to an ethanol/ricin oil molar ratio of 9.86, 1.2 wt% of NaOH and 30 °C. According to the model, these conditions are likely to provide the best process response, leading to the highest ethyl-esters yield (97.123 %) and process efficiency. As can be seen, the optimum values for R and C did not correspond with the points considered in the 23 tests of the experimental design.
To validate the statistical model as well as the experimental design, one additional experiment for triplicate was carried out using these optimum conditions (see Table 5). The low values for the relative error and the standard deviation of the media indicated a considerable agreement between the experimental and the predicted values for the response variable at its optimum point, and this confirmed that the quadratic model was suitable for predicting the course of the ethanolysis of ricin oil. The optimum ethyl-esters yield obtained in this study is higher than the results reported by Plenz et al., [12-13] for the basic ethanolysis of ricin oil with NaOH. Additionally, in this work the reaction was conducted at a lower temperature and in a lesser reaction time.
Table 5. Validation of the statistical model at the optimum conditions
3.3 Ethyl-esters characterization
The basic properties of the ricin oil ethyl-esters produced according to the reaction conditions determined by the RSM are shown in Table 6.
Table 6. Basic properties of ricin oil ethyl-esters
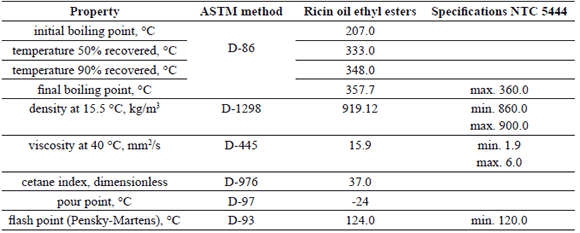
As expected, in agreement with the presence of the OH group in the structure of the ricinoleic acid, the density and viscosity of the ricin oil ethyl-esters are higher than the values corresponding to methyl-esters and ethyl-esters produced from conventional vegetable oils, and also are higher than the specifications taken into account in the Colombian biodiesel quality standard (ICONTEC NTC 5444). These results indicate that ricin oil ethyl-esters can not be used as a neat fuel in modern direct-injection diesel engines. However, they can be used when blended with conventional diesel fuel at low proportions, and also with other kind of biodiesels in order to improve their cold flow properties.
Ricin oil ethyl esters have other potential uses different from fuel for diesel engines. They can be valuable raw materials for oleochemical processes to obtain products with higher added value.
4. CONCLUSIONS
The RSM was successfully used to determine the optimum conditions for the ethanolysis of ricin oil. A quadratic model, established in terms of ethanol/oil molar ratio, NaOH concentration, and reaction temperature, was proven to be very appropriate for predicting the yield of this chemical reaction.
The model was able to predict 99.663 % of the total variation of the system. The optimum experimental conversion was 93.64 %, obtained with an alcohol/oil molar ratio of 9.86/1, a catalyst concentration of 1.2 % with respect to oil weight, and a reaction temperature of 30 °C, for a period of 1 hr. The value of the optimum reaction yield predicted by the model (97.123 %) was close to its actual value obtained through experimentation (93.64 %).
ACKNOWLEDGEMENTS
The authors wish to acknowledge the financial support of DIME (Dirección de Investigación, Universidad Nacional de Colombia, Sede Medellín) to the research project.
REFERENCIAS
[1] MEHER, L. C., SAGAR, D. AND NAIK, S. N. Technical aspects of biodiesel production by transesterification- a review, Renewable and Sustainable Energy Reviews, 10, 248-268, 2006. [ Links ]
[2] SRIDHARAN, R. AND MATHAI, I. M. Transesterification reactions, J. Scient. Ind. Res., 33, 178-187, 1974. [ Links ]
[3] FREEDMAN, B., PRYDE, E. H. AND MOUNTS, T. L. Variables affecting the yields of fatty esters from transesterified vegetable oils, JAOCS, 61(10), 1638-1643, 1984. [ Links ]
[4] MA, F. AND HANNA, M. A. Biodiesel production: A review, Bioresource Technology, 70, 1- 15, 1999. [ Links ]
[5] STAMENKOVIC, O. S., LAZIC, M. L., TODOROVIC, Z. B., VELJKOVIC, V. B. AND SKALA, D. U. The effect of agitation intensity on alkali-catalyzed methanolysis of sunflower oil. Bioresource Technology, 98 (14), 2688-2699, 2006. [ Links ]
[6] SCHWAB, A. W., BAGBY, M. O. AND FREEDMAN, B. Preparation and Properties of diesel fuels from vegetable oils, Fuel, 66, 1372- 378, 1987. [ Links ]
[7] SCHUCHARDT, U., SERCHELI, R., AND VARGAS, R. M. Transesterification of vegetable oils: a review. Journal of Brazilian Chemical Society, 9, 199- 10, 1998. [ Links ]
[8] JOELIANINGSIH, M., H., HAGIWARA, S., NABETANI, H., SAGARA, Y., SOERAWIDJAYA, T. H., TAMBUNAN, A. H. AND ABDULLAH, K. Biodiesel fuels from palm oil via the non-catalytic transesterification in a bubble column reactor at atmospheric pressure: A kinetic study, Renewable Energy, 33, 1629-1636, 2008. [ Links ]
[9] DEMIRBAS, A. Studies on cottonseed oil biodiesel prepared in non-catalytic SCF conditions, Bioresource Technology, 99, 1125-1130, 2008. [ Links ]
[10] LANG, X., DALAI, A. K., BAKHSHI, N. N., REANEY M. J. AND HERTZ P. B. Preparation and characterization of bio-diesels from various bio- oils, Bioresource Technology, 80, 53-62, 2001. [ Links ]
[11] FILLIÈRES, R. B., BENJELLOUN, M. AND DELMAS, M. Ethanolysis of rapeseed oil: quantification of ethyl-esters, mono-, di, and triglycerides and glycerol by high-performance size exclusion chromatography, Journal of the American oil chemist society, 72 (4), 427-432, 1995. [ Links ]
[12] PLENTZ, S. M., MENEGHETTI, M. R., MENEGHETTI, C. R., WOLF, E. C., SILVA, G. E., LIMA, M., COIMBRA, J. I., SOLETTI, M., AND CARVALHO, S. Ethanolysis of castor oil and cottonseed oil: A systematic study using classical catalyst, JAOCS, 83 (9), 819-822, 2006. [ Links ]
[13] PLENTZ, S. M., MENEGHETTI, M. R., MENEGHETTI, C.R., WOLF, G.E., LIMA, L., SILVA, T., SERRA, F., CAUDURO, AND DE OLIVEIRA, L. G. Biodiesel from castor oil: A comparison of the ethanolysis versus methanolysis, Energy & Fuels, 20, 2262-2265, 2006. [ Links ]
[14] VICENTE, G., COTERON, A., MARTINEZ, M. AND ARACIL, J. Application of the factorial design of experiments and response surface methodology to optimize biodiesel production, Bioresource Technology, 8, 29-35. 1997. [ Links ]
[15] HASS, M. J., MARMER, K. M. AND FOGLIA, W. N. In situ alkaline transesterification: An effective method for the production of fatty acid esters from vegetable oils. JAOCS, 81, 83-89, 2004. [ Links ]
[16] RASHID, U. AND ANWAR, F. Production of biodiesel trough optimized alkaline-catalyzed transesterification of rapeseed oil, Fuel, 87, 265-273. 2007. [ Links ]
[17] DOMINGOS, A. K., SAAD, E. B., WILHELM, H. M. AND RAMOS, L. P. Optimization of the ethanolysis of Raphanus sativus (L. Var) crude oil applying the response surface methodology. Bioresource Technology, 99 (6), 1837-1845, 2008. [ Links ]
[18] GHADGE, S. V. AND RAHEMAN, H. Process optimization for biodiesel production from mahua (Madhuca indica) oil using response surface methodology, Bioresource Technology, 97, 379-384, 2006. [ Links ]
[19] SHIEH, C. J., LIAO, H. F. AND LEE, C. C. Optimization of lipase-catalyzed biodiesel by response surface methodology. Bioresource Technology, 88, 103 -106, 2003. [ Links ]














