Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353versão On-line ISSN 2346-2183
Dyna rev.fac.nac.minas v.78 n.170 Medellín dez. 2011
MOISTURE ADSORPTION ISOTHERMS IN YELLOW PITAHAYA (Selenicereusmegalanthus)
ISOTERMAS DE ADSORCIÓN DE HUMEDAD EN PITAHAYA AMARILLA (Selenicereusmegalanthus)
ALFREDO AYALA APONTE
Doctor en Ciencia y Tecnología de alimentos, Profesor, Escuela de Ingeniería de Alimentos, Universidad del Valle, Cali, alfayala04@gmail.com
LILIANA SERNA COCK
Doctora en Ingeniería de alimentos, Profesora, Facultad de Ingeniería y Administración, Universidad Nacional de Colombia, Sede Palmira, lsernac@palmira.unal.edu.co
GLORIA RODRIGUEZ DE LA PAVA
Magister en Ingeniería de alimentos. Profesora, Universidad de San Buenaventura, Cali, gcrodrig@usbcali.edu.co
Received for review April 11th, 2011, accepted July 7th, 2011, final version August, 3th, 2011
ABSTRACT: Moisture adsorption isotherms for yellow pitahaya fruits were studied at 15, 25, and 35 °C using the gravimetric method for a 0.111-0.901 water activity range. Experimental values were adjusted by using the GAB, BET, Henderson, Smith, Caurie, and Peleg models. The isosteric heat of adsorption (Qst) was determined using the Clausius-Clapeyron equation. The results showed that yellow pitahaya fruits exhibit type III adsorption isotherms and that the equilibrium moisture content (EMC) is temperature-dependent. For the same water activity value, the EMC decreased when temperature increased. The GAB model presented the best fitto the experimental values. The Qst dropped from 57.154 to 45.79 kJ/mol when the EMC increased from 0.05 to 0.40 g water/g dry matter, respectively. These results are important for establishing the best storage conditions for dehydrated pitahaya fruits as well as for shelf-life prediction and the design of packaging materials.
KEYWORDS: Yellow pitahaya, adsorption isotherms, isosteric heat of adsorption
RESUMEN: Se determinaron las isotermas de adsorción de humedad en pitahaya amarrilla a 15, 25, y 35ºC mediante el método gravímetro en el intervalo de actividad de agua (aw) entre 0.111 y 0.901. Los valores experimentales de adsorción se ajustaron mediante los modelos de GAB, BET, Henderson, Smith, Caurie, y Peleg. El calor isostérico de adsorción (Qst) se determinó mediante la ecuación de Clausius-Clapeyron. Los resultados mostraron que las isotermas fueron de tipo III. El contenido de humedad de equilibrio (CHE) presentó dependencia con la temperatura, disminuyendo con el aumento de esta para un valor constante de aw. El modelo GAB fue el que presentó mejor ajuste de los valores experimentales. El Qst disminuyó con el aumento del CHE, variando de 57.154 a 45.79 kJ/mol para humedades de 0.05 y 0.4 (g agua/g ms), respectivamente. Estos resultados son de interés para establecer las mejores condiciones de almacenamiento de la pitahaya deshidratada, así como la predicción de la vida útil, y el diseño de empaque.
PALABRAS CLAVE: Pitahaya amarilla, isotermas de adsorción, calor isostérico
1. INTRODUCTION
Yellow pitahaya (Selenicereusmegalanthus), a tropical plant native to Central and South America, belongs to the Cactaceaefamily. Its fruit has yellow skin and a sweet and aromatic white flesh with small black seeds. Ayala et al. (2009) [1] describe the pitahaya as an oval-shapedexotic fruit with exquisite taste and exuberant color. Growing exports in Colombia are driven by higher demand in Europe and the Middle East and by the recognizednutraceuticalfruit properties [2]. Pitahaya is a source of glucose, fructose, dietary fiber, vitamins, and minerals [3,4].The preservation and stabilization of a food's main propertiesduring storage (e.g., its texture and microbiological stability), often require control of moisture content or water activity. Moisture content is an important criterion for judging food quality [5], and water activity (aw) is an essential additional parameter for describing water availability and mobility in foods [6]. Establishing the relationship between equilibrium moisture content (EMC) and aw, also known as sorption isotherm (adsorption or desorption), is important for understanding the stability of foodstuffs [7].
The adsorption isotherm of a food describes the thermodynamic equilibrium state of water. It can be used to predict the shelf life of packaged moisture-sensitive products by modeling moisture uptake during food storage and distribution [8]. It can also be used to determine the best storage methods, packaging materials, and ingredient selection [9].
Adsorption isotherms have been mathematically described through various models, including the theoretical GAB and BET models [10,11] and the empirical or semi-empirical Smith and Oswine models[6]. The GAB and BETequations predict the moisture content of the monolayer(xo) and are considered to be the most useful ones for determining the optimal moisture conditions for food stability during storage [5]. The term xo is the amount of water (g water/g dry matter) that is strongly associated withall active sites of the adsorbent solid phase and its value is correlated to the stability of foods during storage.
The determination of adsorption isotherms at different temperatures also provides information on the thermodynamic properties of the food-water vapor system.Isostericheat of sorption (Qst, kJ/mol), also called differential enthalpy of sorption, is a thermodynamic property that describes the binding strengthbetween water molecules and the food surface. The isosteric heat of sorption is greater than the latent heat of the vaporization of pure water at a given temperature [12]. According to Rizvi (1995) [13],the Qst is a useful parameter in food processes where water desorption or adsorption occurs, because it measures the energy required to break or promote the association between water vapor molecules and adsorbent surfaces, respectively.
No prior sorption isotherms have been reported for yellow pitahaya fruits;therefore, the purpose of this study was to experimentally determine moisture adsorption isotherms in dry samples of yellow pitahaya fruits at 15, 25, and 35 ºC; to adjust known fundamental and empirical models; and to determine the isosteric heat of adsorption at different equilibrium moisture contents.
2. MATERIALS AND METHODS
Plant material
Yellow pitahaya fruits with a maturity stage of three (16-18 °Brix) were selected according to the NTC 3554 standard classification [14]. Fruits were obtained from orchards located in the northern department of Cauca Valley (Colombia), belonging to the Association of PitahayaProducers (ASOPPITAYA).
Experimental procedure
In order to determine the adsorption isotherms of yellow pitahaya fruits at 15, 25, and 35 ºC, the gravimetric static method with saturated saline solutions was used [15]. Eight saturated saline solutions (LiCl, CH3COOK, MgCl2, K2CO3, Mg(NO3)2, NaCl, KCl, and KNO3) with water activity varying from 0.123 to 0.928 at different temperatures were used, as reported by Greespan (1977)[16]. Each saturated solution (35 mL) was placed in a hermetically closed glass container (6 cm height and 5 cm diameter). Rectangular pieces of lyophilized yellow pitahaya (15 mm long x 5 mm wide x 5 mm high, 1.201±00.1 g) were placed in the containers and hermetically closed.The samples were dried in a tray freeze dryer (Labconco, USA) from -35 to 35 °C at 8 Pa vacuum pressure. To avoid microbial contamination, pure Tymol was added to the containers with saline solutions havingan aw greater than 0.65. Each hermetically closed container holding the pitahaya samples was then placed in an environmental controlled chamber (Hotpack, USA) set at 15, 25, or 35 ºC. The samples were weighed every 3 days using a fourth-digit precision analytical balance (Model Mettler-Toledo, Switzerland) until a constant weight (±0.001) was observed, indicating an equilibrium between the samples and the saline solutions. Once the equilibrium was reached (after around 17 to 25 days), the moisture content of the pitahaya fruit slices was determined following the AOAC method 934.06 [17]. The adsorption experiments for each temperature were conducted in triplicate. Analysis of variance for the effect of temperature and water activity on EMC was done as a completely randomized design using Matlab software (V 7.0, Mathworks, Inc., Natick, USA).
Modeling experimental values by sorption models
Six models were used to adjust the EMC experimental values: Guggenheim, Anderson and de Boer (GAB);Brunauer, Emmett, and Teller (BET);and Henderson, Smith, Caurie, and Peleg (Table 1). These models are widely used in the scientific literature in food products [18,19,20]. Model parameters were calculated by using non-linear regression in Matlab software.
Table 1. Models used to predict moisture sorption isotherms in yellow pitahaya
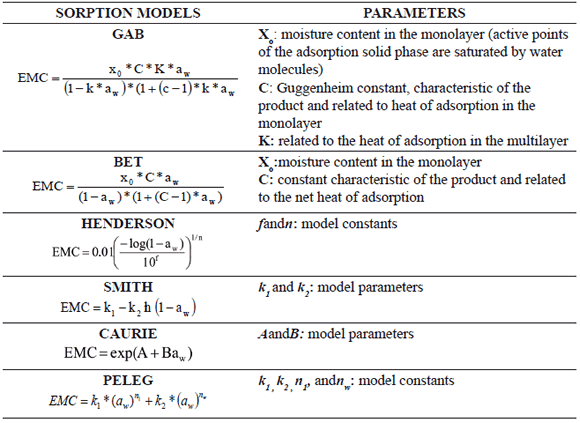
To select the model that best fitted the experimental values, the mean relative error [MRE, %; (1)] and the coefficient of determination(r2) were calculated. A sorption model was considered acceptable when MRE<10% [21] and r21.
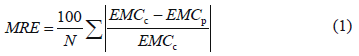
where EMCc=experimental equilibrium moisture content, EMCp=predicted equilibrium moisture content, and N= number of samples.
Isosteric Heat of Adsorption
The isosteric heat of adsorption (Qst), also known as differential enthalpy related to the adsorption process, was determined by adjusting the experimental values to the Clausius-Clayperon equation [22] (2):
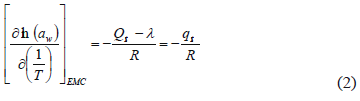
whereqst = net isosteric heat of adsorption, R = universal gas constant, l =latent heat of vaporization of pure water (determined at a fixed temperature of 25 °C, corresponding to an average of 15, 25, and 35°C), aw = water activity, T = temperature, andQst = isosteric heat of adsorption. Plots of ln (aw) vs 1/T at different moisture values resulted in simple linear regression with slope equal to -(Qst-l) /R, from which Qst was determined. Plots of ln (aw) vs 1/T were obtained by using the GAB models to predict aw values at equilibrium moisture contents ranging from 0.05 and 0.40 g water/g dry matter.
3. RESULTS AND DISCUSSION
Moisture adsorption isotherms
Figure 1 shows the experimental moisture adsorption isotherms of pitahaya fruit samples kept at 15, 25, and 35 °C, respectively. The moisture content of the lyophilized pitahaya samples was 1.620± 0.002 g water/g dry matter and the standard deviation of the experimental EMC ranged from 0.0015 to 0.0026. The observed isotherm patterns were classified as type III according to Brunauer et al. (1940) cited by Iguedjtal et al. (2008) [6]. Type III isotherms are characteristic of foods rich in soluble components such as sugars [13]. Other fruits, such as grapes and apricots [23], strawberries [24], kiwis [25], bananas [8], and figs [26] were also reported as exhibiting type III isotherms. The adsorption isotherms showed that with aw up to 0.55, fruits gained relatively low moisture. However, with aw values higher than 0.55, solids solubilization and adsorption promoted a significant increase in moisture content [27,28].
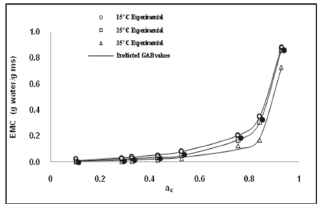
Figure 1. Experimental and GAB predicted adsorption isotherms at 15, 25, and 35 ºC
Analysis of variance (ANOVA) showed a significant effect of aw on EMC (p<0.001). For all isotherms, EMC increased as the aw value increased, being more evident when aw values were higher than 0.55, as mentioned. This is a common pattern in food adsorption processes. A significant effect of temperature on EMC was also observed (p<0.018). When the temperature increased, the EMC decreased for a given aw value. These results indicate that dry pitahaya fruits were less hygroscopic at higher temperature. The same effect has been observed in other fruits such as lychee [29] and grape products (i.e., pestil grape) [19]. Mazza (1980) [30], concluded that an increase in sorption temperature causes physical and chemical changes in the product leading to a reduction in the number of active sites where water molecules bind to the surface of the food. Al-Muhtaseb et al. (2004) [31], showed the dependence between EMC and sorption temperature, resulting in chemical and microbiological reactions associated with food deterioration. An increase of temperature for a given EMC in the food material results in a higher aw, and consequently, in an increase of deterioration rates [10].
Modeling experimental values of sorption
Adjusted parameters and goodness of fit criteria for the various models are shown in Table 2 for the three temperature conditions (15, 25, and 35 °C). Following the criteria ofLomauro et al. (1985) [21] for the goodness of fit of sorption models (i.e., MRE less than 10% and r2 close to 1), the GAB model showed the best fit to the experimental data, with MRE ranging from 1.28 to 2.98% and r2 from 0.9994 to 0.9996 for the three temperature conditions (Figure 1). The Smith model showed the least fit to the experimental values with MRE ranging from 45.01 to 180.38% and r2 from 0.7927 to 0.8853. The GAB model was also reported to fit experimental sorption isotherms in fruit foods such as lemon peel [32],murici and inga[33], grapes [23], and orange leaves [34].
Table 2. Parameter adjustment and statistical criteria of each adsorption model describing equilibrium moisture content vs water activity in lyophilized pitahaya at 3temperatures
The GAB model also offers valuable information on the moisture content of the monomolecular layer (xo), a parameter essential to define storage food conditions (Table 2). The xo indicates the amount of water that is strongly adsorbed in the active sites of the solid phase surface of foods and it is considered to be the moisture content where a given food exhibits its best stability during storage. The xo calculated with the GAB model dropped from 0.0602 to 0.0252 g water/g dry matter, with increasing temperatures. Similar results were found in the adsorption isotherms of fruits such as loquat and quince [35], and pine seeds [36]. The negative relationship between xo and temperature is probably a consequence of damage of the active union points between water molecules and the solid phase surface of the food material, resulting in the detachment of water molecules and therefore in water loss as temperature increases. Moreira (2008) [35], and AviaraandAjibola (2002) [37] stated that when temperature increases, the active points on the food surface are reduced as a result of chemical and physical changes.
The GAB model also contains parameters C (Guggenheim constant) and K, related to the heat of sorption of monolayer and multilayer molecules, respectively (Table 2). Parameter C was reduced from 1.771 to 1.003 with increasing temperature, indicating that the bonding energy for water molecules in the monolayer exhibited an inverse relationship with temperature. Parameter K showed little variation with temperature, and values slightly higher than one, contrary to most reports which showedK values lower than one. In cases where K is larger than one, the isotherm tends to infinity withtheaw value closer to 1.0[38], which was observed in pitahaya fruits when aw values were closer to 0.9 (Fig. 1).
Isosteric heat of sorption
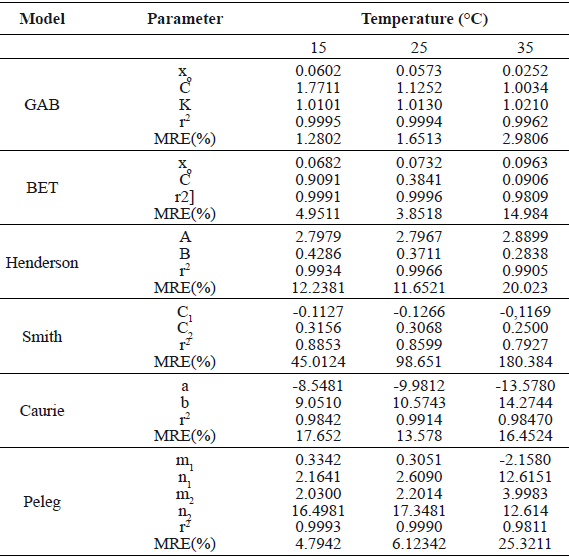
The GAB model was used to predict aw values at various equilibrium moisture content levels. Figure 2 shows the linear representation of -ln(1/aw) against 1/T of the Clausius-Clayperon equation (Eq. 2) for the calculation of Qst during moisture adsorption in pitahaya fruits. The coefficient of determination (r2) was larger than 0.983.
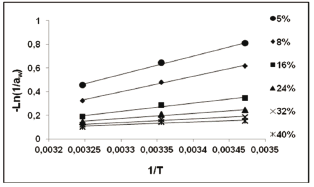
Figure 2. -Ln(aw) vs 1/T for the isosteric heat of adsorption determination in pitahaya fruit
Figure 3 shows the change of Qst as affected by EMC in pitahaya fruits. A close dependency between Qst and EMC was observed. The value Qst dropped from 57.154 to 45.790 kJ/mol when EMC increased from 0.05 to 0.40 g water/g dry matter, respectively. At low EMC levels, more solid-water interactions occur in the active sites of the product surface. However, at a high EMC, the Qst decreases because water occupies less active sites, causing a reduction in the bond energy between water molecules and food components (union forces are reduced). Similar behavior was found in adsorption isotherms for tropical fruits from Asia [29], plantain pulp [39], and other foods such as cakes [12], and milk powder [40].
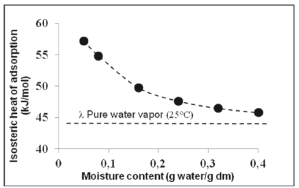
Figure 3. Variation of theisosteric heat of adsorption with the equilibrium moisture content of pitahaya fruit
Cenkowski et al. (1992) [41] suggested that an increase in heat of sorption at low EMC is possibly due to a strong water movement resistance from the interior surface of foods. Other authors argued that highly active polar sites that bind a monomolecular layer of water possibly exist on the solid phase of foods, and that a high amount of energy is required to eliminate this water from foods [35]. The Qst values for all EMC were higher than the heat of vaporization of water (l(25 ºC)=43.961 kJ/Kmol), indicating that the interaction energy for the water molecules on the adsorption sites (active points) of solid foods was larger than the energy necessary to evaporate pure water molecules. Equation 3 shows the relationship between Qst and the equilibrium moisture content during water adsorption in dried pitahaya fruit:
4. CONCLUSIONS
The adsorption isotherms of pitahaya fruits at 15, 25, and 35 ºC exhibited type III behavior, characteristic of foods rich in soluble compounds (e.g., sugars). Our results demonstrate that there is a strong inverse relationship between equilibrium moisture content and temperature, at constant aw values. Pitahaya fruits are less hygroscopic at higher storage temperatures. The GAB model showed the best fit to the experimental adsorption data and was the most appropriate for predicting the equilibrium moisture content of pitahaya fruits between 15 and 35 °C. The Qst decreased from 57.154 to 45.790 kJ/mol when equilibrium moisture content exponentially increased from 0.05 to 0.40 g water/g dry matter, respectively. Adsorption isotherms for pitahaya fruits can be used in the design of optimal storage conditions, shelf-life prediction, packaging materials, and better food mixing operations.
REFERENCES
[1] Ayala, A. A., Serna-Cock, L. and Giraldo, C. J. G., Efecto de la agitación sobre la deshidratación osmótica de pitahaya amarilla (SelenicereusMegalanthus S.) empleando soluciones de sacarosa. Interciencia, 34(7), pp. 492-496, 2009. [ Links ]
[2] Rodriguez, J. P., Narváez, C. E. and Restrepo, L. P., Polygalacturonaseactivity in yellowpitahayapeel (Acanthocereus pitajaya). Acta Biológica Colombiana, 11 (l), pp. 65-74, 2006. [ Links ]
[3] Barbeu, G., The strawberry pear, a new tropical fruit. Fruits, 45, pp. 141-147, 1990. [ Links ]
[4] Wu, M. C. and Chen,C. S., Variation of sugar content in various parts of pitahaya fruit. Proc. Fla. State Hort. Soc., 110, pp. 225-227, 1997. [ Links ]
[5] Arslan, N. and Togrul, H., The fitting of various models to water sorption isotherms of tea stored in a chamber under controlled temperature and humidity, Journal of Stored Products Research, 42(2), pp. 112-135, 2006. [ Links ]
[6] Iguedjtal, T., Louka, N. and Allaf, K., Sorption isotherms of potato slices dried and texturized by controlled sudden decompression. Journal of Food Engineering, 85(2), pp. 180-190, 2008. [ Links ]
[7] Labuza, T. P. and Hymann, C. R., Moisture migration and control in multi-domain foods. Food Science and Technology, 9, pp. 47-55,1998. [ Links ]
[8] Yan, Z., Sousa, M. J. and Oliveira, A. R.,. Sorption isotherms and moisture sorption hysteresis of intermediate moisture content banana. Journal of Food Engineering, 86(3), pp. 342-348, 2008. [ Links ]
[9] Lahsasni, S., Kouhila, M.and Mahrouz, M., Adsorption-desorption isotherms and heat of sorption of prickly pear fruit (Opuntiaficusindica). Energy Conversion and Management, 45, pp. 249-261, 2004. [ Links ]
[10] Van Den Berg, C. and Bruin, S., Water activity and its estimation in food systems. In: Water Activity: Influence on Food Quality (L.B. Rockland, &F. Stewart, eds.) Academic Press, New York, pp.147-177, 1981. [ Links ]
[11] Brunauer, S., Emmett, P.H. and Teller, E., Adsorption of gases in multimolecular layers.Journal of the American Chemical Society.60(2), pp. 309-319, 1938. [ Links ]
[12] Al-Muhtaseb, A.H., Muhanned, A.H., Megahey, E.K., Mcminn, W.A.M. and Magee, T.R.A., Moisture adsorption isotherms of microwave-baked Madeira cake.LWT - Food Science and Technology, 43(7), pp. 1042-1049, 2010. [ Links ]
[13] Rizvi, S. S. H., Thermodynamics properties of food in dehydration. In Engineering Properties of Foods, 2nd Ed., (M.A. Rao& S. S. H. Rizvi, eds) Marcel Dekker Inc, New York, pp. 223-309. 1995. [ Links ]
[14] ICONTEC, Instituto Colombiano de Normas Técnicas y Certificación. Norma Técnica Colombiana. NTC 3554. Frutas frescas. Pitahaya amarilla. Bogotá, pp.1-14, 1996. [ Links ]
[15] Wolf, W., Spiess, E. C. and Jung, G., Standardization of Isotherm Measurements. In: Properties of Water in Foods (D. Simatos& J. L. Multon, eds) MartnusNijhott Publishers: Dordrecht. Netherlands, pp. 661-677, 1985. [ Links ]
[16] Greespan, L., Humidity Fixed Points of Binary Saturated Aqueous Solutions. Journal of Research of the National Bureau of Standards-Physics and Chemistry, 81(1), pp. 89-96, 1977. [ Links ]
[17] AOAC., Official methods of analysis of the Association of Oficial Analytical Chemists International. Moisture in dried fruits.Method 934.06. Arlington, USA, pp. 911-912. 1990. [ Links ]
[18] Jain, S. K., Verma, R. C., Sharma, G. P. and Jain, H. K., Studies on moisture sorption isotherms for osmotically dehydrated papaya cubes and verification of selected models. Journal of Food Science and Technology,47(3), pp.343-346, 2010. [ Links ]
[19] Kaya, S., andKahyaoglu, T., Thermodynamic properties and sorption equilibrium of pestil (grape leather). Journal of Food Engineering, 71(2), pp. 200-207, 2005. [ Links ]
[20] Vega-Galvez, A., Aravena, E. L. and Lemus-Mondaca, R., Isotermas de adsorción en harina de maíz (Zea mays L). Ciencia eTecnologiadeAlimentos, 26(4), pp. 821-827, 2006. [ Links ]
[21] Lomauro, C. J., Bakshi, A. S. and LabuzaT. P., Evaluation of food moisture sorption isotherm equations. Part 1: Fruit, vegetable and meat products. Lebensmittel-Wissenschaftund-echnologie, 18(2), pp. 111-117, 1985. [ Links ]
[22] Tsami, E., Net isosteric heat of sorption in dried fruits. Journal of Food Engineering, 14(4), pp. 327-335, 1991. [ Links ]
[23] Kaymak-Ertekin, F. and Gedik A., Sorption isotherms and isosteric heat of sorption for grapes, apricots, apples and potatoes.Lebensmittel-Wissenschaft und-Technologie, 37(4), pp. 429-438, 2004. [ Links ]
[24] Moraga, G., Martínez, N.,andChiralt, A., Water sorption isotherms and glass transition in strawberries: influence of pretreatment. Journal of Food Engineering, 62(4), pp. 315-321, 2004. [ Links ]
[25] Moraga, G., Martínez, N. and Chiralt, A., Water sorption isotherms and phase transitions in kiwifruit. Journal of Food Engineering, 72(2), pp. 147-156, 2006. [ Links ]
[26] Farahnaky, A., Ansari, S. and Majzoobi, M., Effect of glycerol on the moisture sorption isotherms of figs.Journal of Food Engineering, 93(4), pp. 468-473. 2009. [ Links ]
[27] Saravacos, G. D., TsiourvasD. A. and Tsami, E., Effect of temperature on the water adsorption isotherms of sultana raisins. Journal of Food Science, 51(2), pp. 381-383, 1986. [ Links ]
[28] Hubinger, M., Menegalli, F. C., Aguerre, R. J. and Suarez, C., Water vapor adsorption isotherms of guava, mango and pineapple. Journal of Food Science, 57 (6), pp. 1405-1407, 1992. [ Links ]
[29] Janjai, S., Lamlert, N., Tohsing, K., Mahayothee, B., Bala, B. K.,and Müller, J., Measurement and modeling of moisture sorption isotherm of litchi (Litchi ChinensisSonn). International Journal of Food Properties, 13(2), pp. 251-260, 2010. [ Links ]
[30] Mazza, G., Thermodynamic considerations of water vapour sorption by horseradish roots. Lebensmittel-Wissenschaft und-Technologie, 13, pp. 13-17, 1980. [ Links ]
[31] Al-Muhtaseb, A.H., Mcminn, W. A. M., and Magee, T.R.A., Water sorption isotherms of starch powders: Part 1: Mathematical description of experimental data.Journal of Food Engineering, 61(3), pp. 297-307, 2004. [ Links ]
[32] García, J. V., Cárcel J. A., Clemente G. and Mulet, A., Water sorption isotherms for lemon peel at different temperatures and isosteric heats. LWT-Food Science and Technology, 41(1), pp.18-25, 2008. [ Links ]
[33] Arévalo-Pinedo , A., Dos Santos, F. L., Salles, Z. D., Zuniga, A. D. G. and Arévalo-Pinedo R., Desorption isotherms for murici (Byrsonimasericea) and inga (Ingáedulis) pulps. Journal of Food Engineering, 76(4), pp.611-615, 2006. [ Links ]
[34] Mohamed, A., Kouhila, M., Jamali, A., Lahsasni, S. and Mahrouz, M., Moisture sorption isotherms and heat of sorption of bitter orange leaves (Citrus aurantium). Journal of Food Engineering, 67(4), pp. 491-498, 2005. [ Links ]
[35] Moreira, R., Chenlo, F., Torres, M. D. and Vallejo, N., Thermodynamic analysis of experimental sorption isotherms of loquat and quince fruits. Journal of Food Engineering, 88(4), pp. 514-521, 2008. [ Links ]
[36] Thys, R. C. S., Noreña, C. P. Z., Marczak, L. D. F., Aires, A. G. and Cladera-Olivera, F., Adsorption isotherms of pinhão (Araucaria angustifolia seeds) starch and thermodynamic analysis. Journal of Food Engineering, 100(3), pp. 468-473, 2010. [ Links ]
[37] Aviara, N. A. and Ajibola, O. O.,Thermodynamics of moisture sorption in melon seed and cassava, Journal of Food Engineering, 55(2), pp. 107-113, 2002. [ Links ]
[38] Chirife, J., Timmermann, E. O., Iglesias, H. A. and Boquet, R., Some features of the parameter K of the GAB equation as applied to sorption isotherms of selected food materials.Journal of Food Engineering, 15, pp. 75-82. 1992. [ Links ]
[39] Ciro, H., Osorio, J. A. and Cortés E. A., Determination of the isosteric heat to plantain pulp (musaparadisiaca) by sorption isotherms. Dyna, 76, pp. 127-134, 2008. [ Links ]
[40] Abdenouri, N., Idlimam, A. and Kouhila, M., Sorption isotherms and thermodynamic properties of powdered milk. Chemical Engineering Communications, 197(8): pp. 1109-1125, 2010. [ Links ]
[41] Cenkowski, S., Jayas, D. S. and Hao, D., Latent heat of vaporization of selected foods and crops.CanadianAgricultural Engineering, 34(3), pp. 281-286, 1992. [ Links ]














