Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353versão On-line ISSN 2346-2183
Dyna rev.fac.nac.minas v.78 n.170 Medellín dez. 2011
FIELD STUDY OF EXPERIMENTAL ANTIFOULING PAINT FORMULATIONS
EVALUACIÓN EN CAMPO DE FORMULACIONES EXPERIMENTALES DE PINTURAS ANTI-INCRUSTANTES
FRANKLIN JARAMILLO ISAZA
PhD., Grupo de Corrosión y Protección, CIDEMAT, Universidad de Antioquia, franklinj@udea.edu.co
JUAN GUILLERMO CASTAÑO
PhD., Grupo de Corrosión y Protección, CIDEMAT, Universidad de Antioquia, jacasta@udea.edu.co
FÉLIX ECHEVERRÍA ECHEVERRÍA
PhD., Grupo de Corrosión y Protección, CIDEMAT, Universidad de Antioquia, fecheve@udea.edu.co
Received for review: November 19th, 2010; accepted: March 23rd, 2011; final version: March 28nd, 2011
ABSTRACT: This work presents the results of field testing of several experimental antifouling paints, which were exposed for up to 471 days to natural sea water in the Caribbean. The testing site was selected in order to obtain very critical conditions; therefore, static panels were immersed in water considered as having an intense biological activity. Paints formulations are based in natural rosin as binder, and cuprous oxide as biocide. Three different plasticizers and binder/plasticizers ratios were employed, obtaining 22 different formulations, which were classified in three different groups according to their biocide leaching rate. Besides this, three commercial products were tested for comparison. Some of the experimental formulations, including paints with low biocide release rate, show as good or better performance than the commercial ones. From the plasticizers employed (chlorinated rubber, vegetable oleic acid, and vegetable oil), it was found that the best results were obtained with formulations prepared with chlorinated rubber. It was also observed that physical chemical water properties might have an important effect on paint efficiency, particularly the presence of particulate matter in the water or important variations of water oxygen content.
KEYWORDS: Antifouling paint, biocide leaching rate, copper oxide, marine corrosion, rosin-based paints
RESUMEN: En este trabajo se presentan los resultados de la evaluación en campo de varias pinturas anti-incrustantes desarrolladas experimentalmente, luego de ser expuestas por 471 días a condiciones naturales de agua de mar en el Caribe. El lugar de exposición fue seleccionado con el fin de obtener condiciones ambientales severas, para este propósito se sumergieron paneles estáticos en agua con alta actividad biológica. Se usaron tres diferentes plastificantes y diferentes relaciones plastificante/ligante, para finalmente obtener 22 diferentes formulaciones que fueron clasificadas en tres diferentes grupos de acuerdo a la velocidad de liberación del biocida. Para efectos de comparación, se emplearon tres productos comerciales. Algunas de las formulaciones desarrolladas mostraron igual o mejor desempeño que los productos comerciales. Los mejores resultados se encontraron cuando se usó caucho clorado como plastificante. Finalmente se encontró que las condiciones físico-químicas del agua tienen un efecto significativo en la eficiencia biocida de las pinturas, particularmente la presencia de material particulado y el contenido de oxígeno.
PALABRAS CLAVE: Pinturas anti-incrustantes, liberación controlada de biocida, óxido de cobre, corrosión marina, pinturas a base de resina rosin
1. INTRODUCTION
The marine environment is well known as one of the most challenging when dealing with material and surface protection. Marine biofouling appears to be one of the major problems found in surfaces exposed to such an environment and it is defined as the undesirable accumulation of organisms on surfaces immersed in sea water. This biological process induces inconvenient effects on ships such as raising the frictional resistance, increasing the frequency of dry-docking operations, the deterioration of the corrosion protection system, and the introduction of non-native species into a given environment [1]. The strategy developed to control this phenomenon in recent decades is mainly the use of a protective organic coating containing particulate biocides. These paints can be classified into three groups according to the mechanism they employ to release the biocide: soluble matrix, insoluble matrix or contact paint, and self polishing paints [2]. The main components (binder and biocides) vary with the paint group and, consequently, the application, behaviour, and duration of the antifouling coating. In most cases, formulations of soluble matrix paints employ binders based on rosins and their derivates together with toxic pigments such as copper, iron, or zinc oxides [2]. The main advantage of this sort of antifouling paint is that they can be readily applied on bituminous-based primers. On the other hand, disadvantages are related with their high sensitivity to atmospheric oxidation, restricting the period between paint application and ship immersion, and weak antifouling activity in stationary conditions seriously reducing ship protection for low speed or inactive operation conditions [1]. Tributyltin (TBT) and its derivates prove to be an effective biocide; nevertheless, the serious pollutant effects on the entire marine environment are also well known. Consequently, a ban on TBT's use in antifouling paints was issued by several countries and international organizations [3]. Copper compounds also present a good performance as antifouling biocides; however, contrary to TBT, they are of natural occurrence in sea water and are required for essential biological processes in animals and plants [1,2]. Because of this, although there are big concerns about the danger of high copper concentrations, especially in marine and sportive harbours [4,5,6,7], it seems that antifouling paints releasing copper ions comply with existing environmental regulations [2]. Due to the limited biocide efficiency of copper-zinc, iron, and titanium oxides have been employed as co-biocides; however, little is known about their performance [1].
Soluble matrix paints are mainly based on rosin, which is a natural resin. Antifouling paints formulated solely with rosin present physical and chemical disadvantages which making the use of plasticizers and co-binders necessary [1,8]. These additional ingredients provide the coating with improved mechanical properties, an adequate binder dissolution rate, and consequently act on the biocide release rate [1]. The Caribbean Sea waters are believed to have particularly suitable conditions for biological activity [9] and, therefore, it is important to study the performance of free-tin soluble matrix antifouling paints, aiming at establishing whether high leaching rate paints are required to protect surfaces immersed in these waters. In the present study, several experimental paints were prepared using different plasticizers and co-binders and then tested for about 16 months under very critical static conditions in a rack in Cartagena Bay (Colombia). For reference, three commercial antifouling formulations were tested under the same conditions.
2. EXPERIMENTAL PROCEDURES
What follows is a characterization of the site of study: Previous to paint preparation an intensive study of the physicochemical properties and biological characteristics of the water (at the site selected to immerse the experimental rack) was conducted in order to better understand the quality and exposure conditions, and consequently to choose the more suitable paint formulations and better design of the rack [10]. The variables taken into account to formulate the experimental antifouling paints were:
- Binder: natural rosin (WW colophony), disproportionated rosin and alkaline resins [11] based both on natural and disproportionated rosin. In order to obtain the alkaline resins, copper, calcium, and calcium/zinc salts were employed.
- Plasticizers: chlorinated rubber 20 cP adequately plasticized, vegetable oleic acid (this has no drying characteristics), and vegetable oil (very reactive, it polymerizes and dries very quickly).
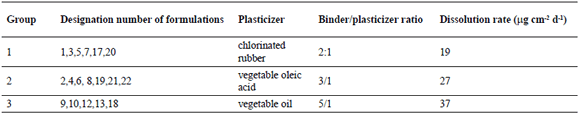
- Binder/plasticizer ratio: In order to range the dissolution rate three ratios were employed 2:1, 3:1 and 5:1.
- Biocide: In all cases, cuprous oxide (Cu2O) was employed as the main toxicant together with zinc oxide (ZnO) in order to reinforce the biocide action. The ratio of cuprous oxide:zinc oxide was 90:10 in weight.
- Extender: Natural calcium carbonate CaCO3 was used both as extender and as a pH regulator at the paint surface/sea water interface.
- Pigment volume concentration: concentrations slightly below the critical value for pigment volume concentration were used in all formulations.
The formulations tested were classified into three groups according to the plasticizer employed and the binder/plasticizer ratio. In Table 1, designations of formulations evaluated and dissolution rates estimated for each group are included.
Table 1. Designation number of formulations and some characteristics of each group of paints evaluated
Leaching rate: Both static and dynamic tests were employed in order to measure the biocide leaching rate, in both cases employing artificial sea water following ASTM D 1141-98. Static tests were carried out by immersing coupons in artificial sea water at room temperature and atomic absorption spectroscopy was employed to periodically measure the copper content in the sea water solution. To control the copper content, the artificial sea water was recirculated, made to pass through an activated carbon filter, and then through an ionic interchange resin to retain part of the soluble copper. Dynamic tests were conducted according to standard procedures included in ASTM D 6442-03.
Panel preparation: Naval steel (ASTM A131) metallic plates were prepared following the ASTM D 3623-78 standard both in dimensions and surface pretreatment. Paints were applied by brush under controlled conditions of temperature and relative humidity. Prior to antifouling paints, a commercial anticorrosive paint system was applied. Dry film thickness of the antifouling paint ranged widely according to paint formulation from 152 to 584 mm; with the lower values for low dissolution rate formulations, and the thicker films for the high dissolution rate formulations. Company recommendations were followed for the application of commercial antifouling paint systems.
Immersion test: A rack was especially designed for the experiment (see Fig. 1) following the recommendations of ASTM D 3623-78, and fabricated in reinforced fibreglass. Design also took into account the results of the physical chemical characterization of the immersion site especially about the depth of samples [10]. Immersion tests took up to about 16 months, and during that period three inspections were carried out. Sandblasted, uncoated, naval steel plates were also exposed with the objective of qualitatively evaluating the test site fouling activity and the attached species.
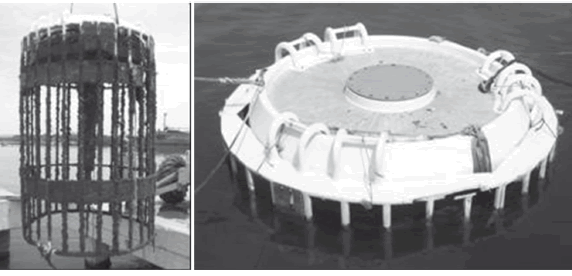
Figure 1. Rack designed for the immersion test
Finally, infrared spectra were collected in the range of 400 to 4000 cm-1 in a Thermo-Nicolet Avatar 330 in the diffuse reflectance mode.
3. RESULTS AND DISCUSSION
In static Cu2O leaching rate tests, the following values for each group were obtained: low rates from 18 to 20 mgcm-2d-1 (Group 1-G1); medium values from 48 to 50 mgcm-2d-1 (Group 2-G2); and finally, high rates ranging from 98 to 100 mgcm-2d-1 (Group 3-G3). The wide range and high leaching rates are due to an intense biological activity and are expected from the conditions (static panels, relatively quiet waters, and high levels of organic matter available) at the test site (9); it is a location at a bay with the rack installed close to the shore and river waters coming into the sea nearby. From the dynamic leaching rate test, and according to the calculations indicated in the ASTM D 6442-03 standard for cumulative release of copper during 45 days of testing, the average values for the formulations in the three groups are: G1: 81 ± 11, G2: 89 ± 6, and G3: 121 ± 19 mg cm-2, in agreement with the measurements obtained by the static procedure, and as expected from the binder/plasticizers ratios employed for the three groups of formulations.
A severe biological activity at the test site was confirmed by the results obtained for uncoated steel plates, which were quickly and profusely colonized in just 50 days of immersion (see Fig. 2). After this period, a clear domain of Mytilopsis sp. and Balanus sp. was observed (see Fig. 3). From the macrofouling formed the occurrence of an epibiosis process can be observed, one of the most frequent mechanisms adopted by organisms in encrusting communities under conditions of either lack of an appropriate substrate or excess of colonizing organisms (9). Other organisms observed were oysters and crabs.
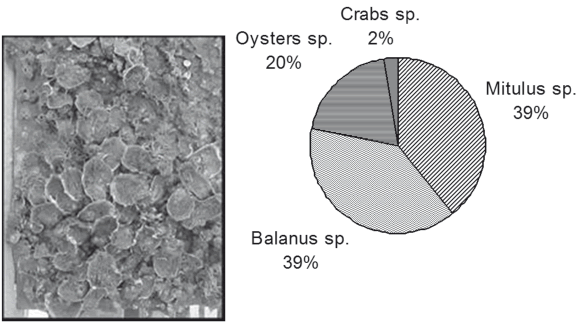
Figure 2. Condition of uncoated steel plates after 50 days of immersion and quantification of marine fouling
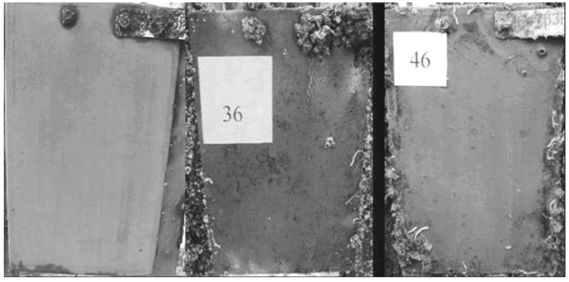
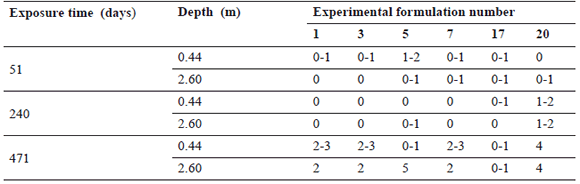
Figure 3. Metallic panels painted with formulation 17 after 51, 240, and 471 days (depth: 2.60 m)
From physical chemical results, it is clear that the photic zone extended up to about 3 m deep from the water surface, and 50 % of transparency was found to be at about 0.44 m deep. According to this result we decided to locate two rows of totally immersed panels, one around 0.44 m, and another just above the photic zone limit, at 2.60 m. Other physical chemical variables of the sea water, particularly temperature, pH, and alkalinity maintained approximately constant values across the photic zone. However, variability was observed for redox potential (from about 140 mV at sea level, to nearly 100 mV at a depth of 4 m), salinity (from about 20 mS/cm at sea level, to nearly 35 mS/cm at a depth of 4 m) and dissolved oxygen (from about 10 mg/L at sea level, to nearly 6 mg/L at a depth of 4 m). This data has been discussed elsewhere [9].
A qualitative scale was employed in order to establish the antifouling efficiency of each paint formulation. Accordingly, a value of 0 was assigned for a 100% efficiency paint, which is a clean, no incrusted surface; and a score of 5 corresponds to 0% efficiency-in other words, a completely incrusted surface [8]. It was also considered that an incrustation value of 1, which refers to about 80% efficiency, is the maximum acceptable value for an antifouling paint to be considered efficient. Panels were evaluated in situ and a photographic record was taken. Tables 2 to 5 show the evaluation results after 51, 240, and 471 days of immersion for both experimental formulations and reference commercial paints.
Table 2. Evaluation results after 51, 240, and 471 days of immersion for Group 1 experimental formulations. A value of 0 was assigned to 100 % biocide efficiency paint and a score of 5 corresponds to 0 % efficiency.
From Table 2 it can be observed that most G1 formulations performed well up to the second inspection period, except formulation 20, which failed before 240 days of exposure. Formulation 17 was the only one that resisted the whole test period (see Fig. 3). Small differences were observed between the two depths of testing despite the fact that the samples immersed to 2.60 m were found to be readily covered by mud due to abundant suspended particles in the water.
The mud layer may have a blocking effect on the antifouling action of the paint, so premature failure of the panels exposed at this depth could be expected. This, however, it is not generally observed for panels painted with G1 formulations; only the results for formulation 5 show how, at 471 days, the panel exposed at 2.60 m completely failed, whilst the panel at 0.44 m still performed well. In the formulations in this group, chlorinated rubber was employed in different binder/plasticizer ratios and a couple of them show acceptable resistance protection despite their low leaching rate. This is an important finding since due to environmental policies to have the lowest biocide release rates possible is desirable.
In the case of G2 paints (Table 3), an acceptable performance was observed for formulations 4, 8, 19, and 22 (see Fig. 4). The failure observed in formulation 4 panels exposed at 2.60 m must be related with the mud layer formed on their surface as discussed above, being that the panels for the same formulation exposed to 0.44 m were still in good condition. These formulations employed vegetable oleic acid as a plasticizer, obtaining a higher leaching rate and consequently, a relatively better performance compared with G1 paints. Four out of 7 formulations had an acceptable antifouling resistance during the experiment.
Table 3. Evaluation results after 51, 240, and 471 days of immersion for Group 2 experimental formulations
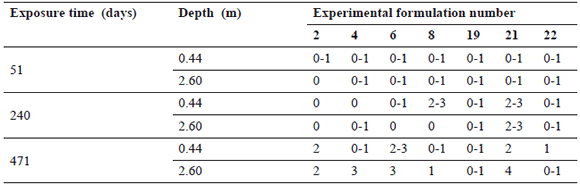

Figure 4. Metallic panels painted with formulation 19 after 51, 240, and 471 days (depth: 2.60 m)
In Table 4, the results of G3 formulations only show how experimental paints 12 and 13 (see Fig. 5) completed the experiment without failing. The worst protection of all was seen by formulations 10 and 18 (see Fig. 6), which failed prematurely.
Table 4. Evaluation results after 51, 240, and 471 days of immersion for Group 3 experimental formulations
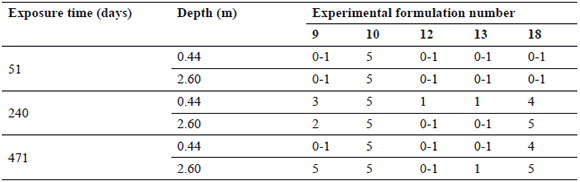
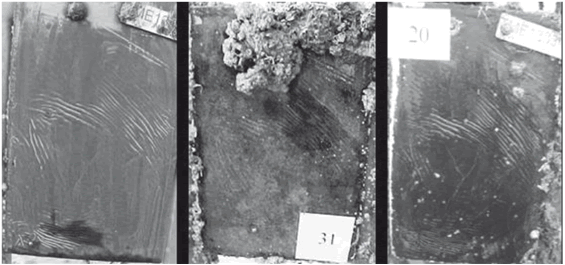
Figure 5. Metallic panels painted with formulation 13 after 51, 240, and 471 days (depth: 2.60 m)

Figure 6. Metallic panels painted with formulation 10 after 51, 240, and 471 days (depth: 2.60 m)
According to the overall results, it might be considered that the experimental paints using vegetable oil as plasticizers are inadequate for use under the selected conditions. This can be stated being that only 2 out of 5 formulations in this group performed well despite their high biocide release rate, making G3 paints the most ineffective of the three groups.
For the commercial formulations (Table 5), only the paint of company 3 resisted the whole period of experimentation, whereas the worst performance of these formulations was exhibited by the paint provided by company 1. In addition, it can be seen that most of the G1 formulations were as good as or even better than paints from companies 1 and 2 (see Fig. 7).
Table 5. Evaluation results after 51, 240, and 471 days of immersion for commercial paints
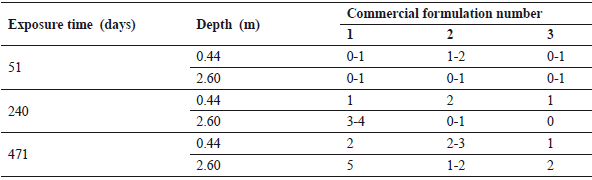

Figure 7. Metallic panels painted with a commercial paint after 51, 240, and 471 days (depth: 2.60 m)
Figure 8 shows the infrared spectra for formulation 8 after 471 days of exposure time at different depths. The typical absorption bands can be observed for calcium resinate at 2922 cm-1, hidrochlorinated aliphatic chains at 2100 and 1900 cm-1, probably after a long exposure time of the resin, carbonyl group at 1630 cm-1, from the tensoactive components of the paint, and two bands at 642 cm-1 and 476 cm-1 associated with Cu2O and ZnO, respectively. Interestingly, the increasing depth results in a gradual decreasing of both calcium resinate (2922 cm-1) and inorganic component bands (642 cm-1 and 476 cm-1) to the point at which they practically disappear at 2.60 m depth, as shown in Fig. 8c. Looking at the water's physical chemical properties which show some variations with depth, the oxygen water concentration appears to be the most influential on the dissolution rate. This concentration is about 70 % higher at sea level, inducing more oxidation of the rosin, and therefore reducing its dissolution rate. This result is in agreement with the literature [1].
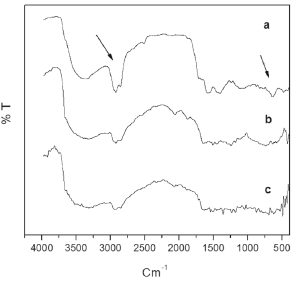
Figure 8. IR spectra for formulation 8 (from G2) after 471 days of exposure time (a) at sea water level, (b) at 0.44 m, and c) at 2.60 m of depth. Arrows denote the calcium resinate band at 2922 cm-1and the Cu2O band at 642 cm-1.
4. CONCLUSIONS
Experimental antifouling paint formulations were developed, which perform at least as good as commercial products. This was achieved even when the paint has a low biocide release rate.
Paints containing chlorinated rubber perform better than those formulated with oleic plasticizers, as indicated by the results of G1 paints compared with those of G2 and G3 formulations.
The testing site conditions might have an important influence on the paint efficiency, as revealed in the present study when interfering suspended matter was present in the water, or due to considerable variations of oxygen content. Therefore, a careful characterization of the water's physical chemical properties at the testing site must be carried out.
ACKNOLEGMENTS
The authors would like to acknowledge the support from "Programa de Sostenibilidad, Universidad de Antioquia"
REFERENCES
[1] Meseguer Yebra, D., Kiil, S., Dam-Johansen K., Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings, Progress in Organic Coatings, 50,pp. 75-104, 2004. [ Links ]
[2] Almeida, E., Diamantino T. C., De Sousa, O., Marine paints: The particular case of antifouling paints, Progress in Organic Coatings, 59, pp. 2-20, 2007. [ Links ]
[3] Konstantinou, I.K., Albanis, T.A., Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: a review, Environment International, 30, pp. 235- 248, 2004. [ Links ]
[4] Schiff, K., Diehl, D., Valkirs, A., Copper emissions from antifouling paint on recreational vessels, Marine Pollution Bulletin, 48, pp. 371-377, 2004. [ Links ]
[5] Katranitsas, A., Castritsi-Catharios, J., Persoone, G., The effects of a copper-based antifouling paint on mortality and enzymatic activity of a non-target marine organism, Marine Pollution Bulletin, 46, pp. 1491-1494, 2003. [ Links ]
[6] Saphier, A. D., Hoffmann, T. C., Forecasting models to quantify three anthropogenic stresses on coral reefs from marine recreation: Anchor damage, diver contact and copper emission from antifouling paint, Marine Pollution Bulletin, 51, pp. 590-598, 2005. [ Links ]
[7] Comber, S.D.W., Franklin, G., Gardner, M.J., Watts, C.D., Boxall, A.B.A., Howcroft, J., Partitioning of marine antifoulants in the marine environment, The Science of the Total Environment, 286, pp. 61-71, 2002. [ Links ]
[8] Del Amo, B., Giudice, C. A., Rascio, V., Influence of binder dissolution rate on the bioactivity of AF paints, Journal of Coatings Technology, 56, pp.63-73,1984. [ Links ]
[9] Echeverría, F., Aguirre, N., Castaño, J. G., Valderrama, A. C., Peña, J. D., Giudice, C., Caracterización fisicoquímica y biológica de la bahía de Cartagena en la zona de Mamonal para la evaluación de pinturas antiincrustantes en condiciones estáticas, Revista Facultad de Ingeniería, 39, pp. 7-20, Noviembre 2006. [ Links ]
[10] Echeverría, F., Castaño, J. G., Aguirre, N., Valderrama, A. C., Peña, J. D., Herrera, L., Giudice, C., Morales, A., Planteamiento metodológico para la evaluación del desempeño de pinturas antiincrustantes en condiciones estáticas, Revista Facultad de Ingeniería, 37, pp. 155-163, Julio 2006. [ Links ]
[11] Meseguer Yebra, D., Kiil, S., Dam-Johansen, K., Weinell, C., Reaction rate estimation of controlled-release antifouling paint binders: Rosin-based systems, Progress in Organic Coatings, 53, pp. 256-275, 2005. [ Links ]














