Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353On-line version ISSN 2346-2183
Dyna rev.fac.nac.minas vol.79 no.171 Medellín Jan./Feb. 2012
RIVERBANK FILTRATION: AN EFFICIENT AND ECONOMICAL DRINKING-WATER TREATMENT TECHNOLOGY
FILTRACIÓN RIBEREÑA: UNA TECNOLOGÍA EFICIENTE Y ECONÓMICA PARA EL TRATAMIENTO DE AGUA POTABLE
MARCELA JARAMILLO
Geóloga, MSc. , PhD. (C) Ingeniería, Recursos Hidráulicos, Universidad Nacional de Colombia, Sede Medellín, mjaramiu@unal.edu.co
Received for review: November 10th, 2010; accepted: March 28rd, 2011; final version: May 12nd, 2011
ABSTRACT: Riverbank filtration (RBF) is a water treatment technology that consists of extracting water from rivers by pumping wells located in the adjacent alluvial aquifer. During the underground passage, a series of physical, chemical, and biological processes take place, improving the quality of the surface water, substituting or reducing conventional drinking water treatment. Despite its extensive use in Europe and its emerging use in the United States, there are no scientific publications related to RBF use in Colombia, although apparently propitious settings exist. The main objective of this paper is to present a brief overview of the theoretical foundations of the technique, its benefits, and limitations.
KEYWORDS: riverbank filtration, aquifer-river interaction, groundwater, water treatment, water quality, water supply, surface and subsurface hydrology.
RESUMEN: La tecnología de filtración ribereña (FRB) consiste en extraer agua de corrientes superficiales mediante el bombeo de pozos ubicados en el acuífero aluvial adyacente al río. En el trayecto se presentan procesos físicos, químicos y biológicos que mejoran la calidad del agua superficial, sustituyendo o reduciendo los tratamientos convencionales de agua para consumo humano. A pesar de su extensivo uso en Europa y su reciente utilización en Estados Unidos, no existe ninguna publicación científica que dé cuenta de su uso en Colombia, a pesar de presentarse escenarios aparentemente propicios. El objetivo principal de este artículo es presentar una breve reseña sobre los fundamentos teóricos de la técnica, sus beneficios, y limitaciones.
PALABRAS CLAVE: filtración ribereña, interacción acuífero-río, aguas subterráneas, tratamiento de agua, calidad de agua, abastecimiento de agua, hidrología superficial y subterránea.
1. INTRODUCTION
Alluvial aquifers are widely used as a groundwater source in many countries, mainly due to their high production potential, proximity to demand areas, their ease, and economy of extraction [1]. By pumping wells located in an alluvial plain hydraulically connected to a river it is possible to generate a hydraulic gradient so that surface water is forced to flow through the bed and the banks of the river (Fig. 1). During this process, known as riverbank filtration (RBF), a reduction in the concentration of pollutants is achieved by physical, chemical, and biological processes that take place, between the surface water and groundwater, and with the substrate [2].
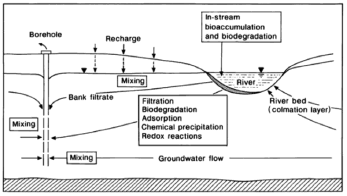
Figure 1. Basic scheme of riverbank filtration and main attenuation processes [5]
The reduction of pollution levels is accomplished by a number of processes including physical filtration, microbial degradation, ion exchange, precipitation, sorption, and dilution [3]. Other factors that also contribute to the treatment are: the river water and the groundwater quality, the porosity of the medium, the water residence time in the aquifer, temperature and pH conditions of water, and oxygen concentrations [4].
In addition to the removal of pollutants (particles, microorganisms, organic, and inorganic compounds, etc.) there are two additional advantages of RBF. The first is relative to the fact that the flow through the aquifer acts as a barrier against concentration peaks that may result from accidental spills of pollutants. The second is the regulation on the temperature variations in the river water: during winter, when air temperatures are low, the filtered water is usually warmer than surface water, and in summer it is cooler. The lowest variation in temperature improves the quality and further processing of the bank filtrate [5].
Riverbank filtration technology has been a common practice in Europe for over 100 years, particularly in countries such as Switzerland where 80 % of drinking water comes from RBF wells, 50 % in France, 48 % in Finland, 40 % in Hungary, 16 % in Germany, and 7 % in the Netherlands [2]. In Germany, for example, 75 % of the city of Berlin depends on RBF, whereas in Düsseldorf RBF has been used since 1870 as the main drinking water supply [6]. In the United States, on the other hand, this technique has been used for nearly half a century, especially in the states of Ohio, Kentucky, Indiana, Illinois, among others [3]. Other countries that have recently started implementing RBF for drinking water supply are India [7], China, and South Korea [8].
All the aspects mentioned above make RBF a very appealing tool to be implemented in a country like Colombia, where a huge percentage of the population does not have access to good quality drinking water. However, no reports of RBF in Colombia have been found in the available scientific literature. This situation is inexplicable since Colombia has apparently propitious settings for the implementation of RBF. It is possible to speculate that RBF is indeed used in Colombia but in an empirical way, or even unconsciously, as it is the case of shallow wells by rivers where some surface water could be being pumped along with groundwater.
The main objective of this paper is to present a brief summary on the theoretical fundaments of RBF technology, its benefits, and limitations.
3. SITING AND DESIGN
Local factors such as river hydrology, hydrogeological site conditions (i.e., aquifer thickness and hydraulic conductivity), and the aims of water withdrawal determine not only the capacity of the wells, but also the travel time of the bank filtrate, and distance between the river and the well [9].
Riverbank filtration wells can be designed either vertically (as the most common practice especially for the extraction of low water quantities) or horizontally (for higher extraction rates). Horizontal wells (sometimes with a radial pattern), also known as collector wells, are usually directed toward the river and extract water from beneath the riverbed, whereas vertical wells extract water along the riverbed [3]. Also, RBF wells can be distributed parallel to the riverbank in galleries or groups [9].
Grischek et al. (2002) [9] compiled available information from RBF systems in the United States and Europe, and concluded that the most important parameters for success during RBF are the flow path length, the thickness of the aquifer, and the infiltration area in the river. Finally, the authors conclude that the siting and design of an RBF system does not only depend on hydrogeological factors, but also on technical, economical, regulatory, and land-use factors.
3. ATTENUATION PROCESSES DURING RBF
Four attenuation processes are involved in RBF: hydrodynamic, mechanical, biological, and physicochemical [1]
Hydrodynamic processes include convective-dispersive transport, and dilution. The aquifer acts as a filter for the temporal variation of the pollutant compounds in the river caused by accidental (or intentional) spills, which, due to the connection between the river and the aquifer, represent a risk of contamination to the groundwater. As a result, high frequency variations in the surface water quality are reduced in groundwater. Beyond smoothing fluctuations in river water quality, dilution takes place when the river water mixes with groundwater, which is usually of higher quality, further enhancing the quality of bank filtrate [1].
The most important mechanical processes for the improvement of water quality are those involving the natural filtration of fine sediments, particulate organic matter, and pathogens, especially in the first few meters from the river to the well [5] [1]. A disadvantage of physical filtration is associated with the obstruction or the clogging of the porous media, as will be explained later.
The biological processes that occur during RBF are directly dependent on the type of microorganisms that inhabit the aquifer [1] [10]. The metabolic processes of these microorganisms mainly determine the final quality of filtered water.
Finally, physicochemical processes are associated with sorption, precipitation reactions, flocculation, coagulation, and redox reactions [1]. All these processes govern the removal of particles from the porous media, affecting the concentration and the behavior of metals and other inorganic compounds, thus having implications for the chemical evolution of water.
Figure 1. summarizes the main attenuation processes during riverbank filtration.
4. CONTAMINANT REMOVAL
4.1 Organic Contaminants
Organic pollutants such as pesticides, herbicides, odorous compounds, oil sub-products, and pharmaceuticals are of great concern for water quality. Riverbank filtration has been extensively used for drinking water pretreatment in places with such pollution problems [11] [12]. The removal and the behavior of organic compounds during RBF depends on factors specific to pollutants such as the hydrophobicity of the compound, the potential for biochemical degradation, the amount of organic matter in the aquifer, microbial activity, infiltration rate, biodegradability, etc. [2]. Another aspect that apparently influences the removal of certain organic contaminants such as antimicrobial residues is the redox condition of the aquifer together with the travel time [13].
Although RBF has proven to be a good pretreatment technique for a large number of organic compounds, it has been found that some compounds, such as certain pesticides, pharmaceuticals, and halogenated organic compounds are more resistant to removal [4] [3] [1].
4.2 Inorganic Compounds
The main and most common processes that control the transport and fate of inorganic compounds in RBF are [2]:
- Redox reactions: manganese and iron oxides are mobilized under reducing conditions and adsorbed, precipitated, or co-precipitated under oxidizing conditions.
- Microbial degradation of organic matter: this can alter the geochemical conditions and mobilize metals usually associated with natural organic matter (NOM) such as copper and cadmium.
- Dilution: high concentrations of inorganic compounds in river water are depleted by the mixing of surface and groundwater.
4.3 Microbial Pathogens
The primary processes for the removal of pathogens during soil passages are inactivation, attachment to the aquifer grains (adsorption), straining, and sedimentation [14]. These processes depend on the climate (temperature, rainfall, etc.), the nature of the porous media (clay content, moisture-holding capacity), and the type of microorganism [15].
4.3.1 Inactivation
Inactivation is a pathogen's loss of ability to infect host cells. This happens with time because of the disruption of coat proteins and the degradation of nucleic acids [16].
Among the main factors that influence virus inactivation rates are temperature, adsorption to particulate matter and soil, and microbial activity [14]. Many authors consider temperature to be the most significant factor for inactivation; however, temperature sensitivity and inactivation speed appear to be virus-dependent [17]. For instance, at normal groundwater temperatures (8 to 25 °C), inactivation rates for pathogens are very low: 1.42/day for Shigella sp. (McFeters et al., 1974), 0.51/day for Salmonella sp. (Keswick et al., 1982), and 0.33/day for E. coli O157 (Rice et al., 1992) (in [14]).
4.3.2 Adsorption
Adsorption is defined as the sum of the electrostatic, hydrophilic, and steric interactions between viruses and the media itself [18]. The interactions that take place between a microorganism (especially a virus) and soil particles depend on their surface characteristics [19]. Those characteristics may be altered by changes in pH, ion strength, multivalent ions, organic matter [16], and temperature [20].
4.3.3 Straining
Straining is the physical removal of microbial particles, and depends on their size and that of the pore throats. According to McDowell-Boyer et al. (1986) [21], if the ratio of the diameter of the media to the diameter of the particle is greater than 20, then the straining is not considerable, but if the same ratio is between 10 and 20 the removal of particles is significant. Below a ratio of 10 there is not penetration of the particle through the porous media.
4.3.4 Settling
Also known as sedimentation in pores, settling is determined by Stoke's settling velocity which states that the settling velocity directly depends on the mass density difference between the particle and the fluid, particle size, and gravity force, and inversely, on the fluid viscosity. In the case of the settling of pathogens in groundwater, the flow velocity is an important factor, as the settling is most likely to occur at low groundwater velocities [14].
Several authors have confirmed the efficiency of RBF in the reduction of pathogenic microorganisms. Wang (2002) [22], for example, reports a 3.8-log (% removal = 100 - 10(2-x), where x is the number of log removal, i.e., 1 log = 90 % removal) reduction for total coliform, and 2.0-log units for HPC bacteria in Louisville, Kentucky. Weiss et al. (2002a) [23] found a removal of 2.9 to 3.4 logs for Clostridium, 2.3 to 3.0 logs for E. coli C, and 1.6 to 2.0 logs for E. coli Famp, in three RBF systems along the Ohio, Wabash, and Missouri rivers in the United States. Also, at the Greater Cincinnati Water Works in Ohio, a minimum of 4-log removal of Giardia and Cryptosporidium was reported by Gollnitz et al. (2003) [24]. During a 24-month study at the Central Wyoming Regional Water System, Gollnitz et al. (2005) [10] found an average of 2.1-log reduction for surrogates of Giardia and Cryptosporidium (total coliform, E. coli, enterococci, total aerobic endospores, algae, diatoms, and turbidity). In Germany, Schubert [25] [26] reports a 5-log removal of bacteria, viruses, and parasites at the Flehe Waterworks in Düsseldorf (which began operation in 1870).
5. THE HYPORHEIC ZONE
The hyporheic zone is defined as the transition zone between surface water and groundwater in the alluvial aquifer. This area experiences biogeochemical activity which is much more intense than surface water or groundwater [27]. This biochemical activity is reflected in the complex and dynamic gradients of light, temperature, pH, redox potential, oxygen content, and organic matter content [2].
The most important biochemical change that takes place in the hyporheic zone is the creation of an anaerobic zone (Fig. 2). This occurs as a result of the rapid oxygen consumption involved in microbial activity associated with the degradation of organic matter and organic contaminants. These anaerobic conditions increase the activity of denitrifying bacteria and sulfate-reducing bacteria, creating a highly reductive area, which causes the dissolution of manganese and iron oxides, thus affecting the quality of filtered water [28].
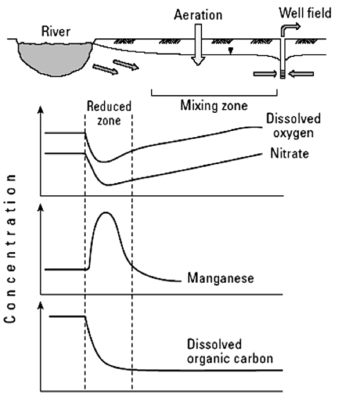
Figure 2. Water chemistry changes in the hyporheic zone [2]
With an increased distance from the river, microbial activity decreases and the oxygen supply coming from the unsaturated zone increases, thus creating an oxidizing environment where manganese and iron are removed from groundwater by precipitation and adsorption to the surfaces of the grains comprising the porous media.
Another consequence of microbial activity in the hyporheic zone is the formation of biofilms that can block the pores of the aquifer and reduce its permeability. Clogging of the pores of the aquifer can also be induced by the retention of fine sediments (< 2 mm) in the hyporheic zone, especially the precipitation of sulfides and oxides [27].
6. RBF VS. CONVENTIONAL WATER TREATMENT
Coagulation-flocculation followed by sedimentation, filtration, and disinfection (Fig. 3), often by chlorination, is used worldwide in the water treatment industry before distributing treated water to consumers [29].
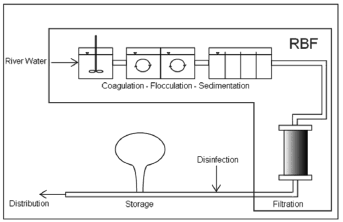
Figure 3. Conventional water treatment process vs. RBF
Based on German experience with bank filtration along the Rhine River, Kuehn and Mueller (2000) [4] compared the processes for the treatment of raw river water versus those needed for bank filtrate, and found that some conventional water treatment processes can be eliminated if RBF water is used, i.e., coagulation/flocculation, sedimentation, and sometimes filtration (Fig. 4).
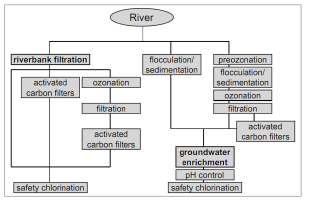
Figure 4. Processes for the treatment of raw river water and bank filtrate in Germany [4]
Bank-filtrate water usually requires additional treatment before disinfection, such as activated carbon filtration (ACF), ozonation → filtration → ACF, or aeration → filtration. This is especially common in rivers with high concentration of ammonia, organic compounds, and micro-contaminants.
The content of dissolved oxygen (DO) in river water is an important factor for determining the need for further bank filtrate treatment before disinfection. For example, if conditions become anaerobic either due to the low DO content in the river water or because of a high oxygen demand due to the presence of microorganisms in the soil, iron and manganese will undergo chemical reduction and solubilize in the water, requiring their removal by further treatment such as aeration and filtration, before disinfection [29].
Ammonia content in river water also determines the quality of bank filtrate [4] and the necessity of performing other treatment processes before disinfection. This is because nitrification (the oxidation of ammonia into nitrites and then into nitrates) is an aerobic process that consumes oxygen in river water. If DO content in river water is already low, then anaerobic conditions will develop, and the iron and manganese which would otherwise be present as precipitated in their oxidized form (Fe3+ and Mn3+), will be solubilized (as explained above) [2].
Biological processes can also contribute to oxygen consumption and thus to the solubilization of iron and manganese. These processes depend on different factors such as pH, temperature, DO, and the content of organic compounds in the water [4].
Kim and Corapcioglu (2002) [31] and Kim et al. (2003) [32] demonstrated that colloidal particles and dissolved organic matter (DOM) present in the water can increase the mobility of contaminants by the reduction of retardation, and that they can change the degree of sorption and microbial degradation. If this happens, the efficiency of RBF will be compromised since those contaminants might be present in the bank filtrate. Depending on the nature and final concentration of the contaminant, the bank-filtrate will need further treatment before disinfection.
When chlorination is used as the disinfection process, chlorine reacts with natural organic matter (NOM) and halides (Cl-, Br-) to form disinfection byproducts (DBPs) such as trihalomethanes (THMs). Those DBPs are harmful for human health, especially brominated trihalomethanes (THMs-Br) which are suspected to be much stronger carcinogens and mutagens than their chloride-containing analogues [33].
Although the RBF has proven to be efficient in removing organic matter (total and dissolved organic carbon, TOC and DOC) as well as certain DBPs [34], if chlorination is used as the disinfection method, there might be an increase in THM concentration. It could then be recommended to use ACF before disinfection to reduce the amount of TOC and thus the formation of THMs.
7. RBF LIMITATIONS
In addition to the inability of RBF to remove certain biological, inorganic, and organic contaminants, limitations associated with the hydrology and dynamics of the river and groundwater cannot be ignored. On the contrary, these aspects should be taken into account when RBF is considered as a pretreatment solution [6].
Changes in the hydraulic gradient from the river to the aquifer, and in the hydraulic conductivity of the alluvial deposits, generate changes in the pore water velocity as well as in the retention time, which may limit or change the biogeochemistry activity that takes place in the hyporheic zone. Finally, changes in water temperature affect not only the hydraulic conductivity due to the reduction of the viscosity of water, but also the rate of biogeochemical processes and microbial activity, which could weaken the final quality of the filtered water [35].
Fluctuations in the river stage alter water saturation, biofilms content, geochemistry, and even the structure of the RBF system, thus affecting the performance of the treatment. Such variations can affect the flow and transport characteristics of the whole system because the unsaturated region, which may not have the same removal potential as the saturated zone, could be infiltrated by river water during an increased stage of water level and, therefore, the filtered water obtained will have a poorer quality [36].
The efficiency and performance of RBF can also be compromised by scouring processes carried out on the river bed and banks when the flow rates are very high, which is a fairly common problem [37]. The consequent loss of fine sediments, responsible for the low permeability of the river bed (usually one to three orders of magnitude below the permeability of the aquifer) could be a problem in the treatment process in such cases, since the efficiency of filtration decreases. Another effect of scouring is the removal of microorganisms which are essential for improving the quality of river water in the hyporheic zone.
Another limitation associated with RBF is the obstruction or clogging of the porous media. There are four types of clogging: mechanical, physical, biological, and chemical.
Mechanical clogging is defined as the blocking of flow through porous media due to the entrapment of gas. Entrapped gas that is released from or dissolved into the porous media can change the permeability of media, thus preventing the water from making its way through the aquifer [38].
Microorganisms produce a range of poorly soluble gases such as carbon dioxide (CO2), nitrogen (N2), hydrogen (H2), oxygen (O2), and methane (CH4). However, O2 and H2 have rarely been reported to accumulate in soil [39]. Nitrogen gas produced by denitrification, and methane produced during methanogenesis have been further identified as the most prevalent gases thus entrapped.
Physical clogging is caused by the continual percolation of river water containing suspended matter due to well pumping [40]. This process is governed by the dynamics of the river (runoff, erosion, transport, and sedimentation), the quality of the river water, the location of the wells related to river geomorphology, and the distance between the well and the riverbank [41].
The settling velocity of fine particles inside the aquifer pores depends not only on the physical properties of the sediments (size, weight, etc.), but also on the water velocity (river discharge) and viscosity [42]. This means that a particle will settle easier on a low-flow low-viscosity river.
Sometimes reductions on the hydraulic conductivity of the aquifer (manifested by increasing drawdown) are related not only to clogging, but also to low water temperatures [43] [44].
Hubbs (2006a) [42] presents another possibility for mechanical clogging that has to do with the development of unsaturated conditions under the riverbed. In that case, the forces of the overlying water column compress the loose soil into a structure with much lower hydraulic conductivity.
Biological clogging is caused by excessive biomass accumulation in the riverbed [45]. The distribution of biomass has been assumed to be uniform like a biofilm covering the grain surfaces; however, it has been found that microorganisms grow in micro-colonies, and that plugs of biomass, rather than biofilms, are responsible for bioclogging [46].
Biofilms are the normal environment for most microbial cells (especially bacteria) in many natural and artificial habitats. They are an aggregation of microorganisms growing in a matrix of expolysaccharides, by adhering either to each other or to a grain surface [47]. The production of polysaccharides can be favored by factors such as temperature, redox potential, the availability and nature of organic substrate, nitrogen availability, O2 concentration, and the physiological status of microorganisms [39].
Due to the fact that each type of microorganism has its own optimum growth temperature [14] the extension and formation rates of biofilms in a riverbed will depend highly on the temperature of the water infiltrating the riverbed.
Chemical clogging is caused by the precipitation of compounds into the pores of the aquifer. Some factors thought to influence chemical clogging are iron, ammonia, and nitrate concentrations, and the hardness of the water [48].
High loads of biodegradable substances in the river water can lead to chemical clogging due to strong changes in redox-potential and pH values, which may cause the precipitation of substances into the pores of the aquifer [6]. These changes are strongly related to the microbial activities in the riverbed, since it controls the redox conditions of the medium to a high degree, preventing or stimulating the precipitation of inorganic substances [48]. That is the reason why some authors refer to biochemical clogging.
In general, biochemical clogging occurs below the infiltration area where mechanical clogging predominates [41].
Clogging may be limited or removed by the self cleaning potential of the river: scouring [6]. The scouring process is the result of the shear forces imparted by the movement of water flowing into the river, and the resistance to the movement provided by the riverbed itself, which is a function of the river slope, the vertical velocity profile, and sediment transport [42]. However, in some cases, larger sediments form a sort of armor that prevents sediment resuspension from the bed by the action of flood waves [49].
8. CONCLUSIONS
During RBF, pumping pressure in the alluvial aquifer adjacent to the river will force the water to percolate from the river into the aquifer. In this path, a series of physical and biogeochemical processes take place, including physical filtration, adsorption, absorption, biodegradation, and dilution. Thus, riverbank-filtrate often shows better quality than river water, making its treatment for human consumption a lot easier and less expensive.
The removal of sediment, organic and inorganic compounds, and pathogens takes place during the first meters from the river in what is known as the hyporheic zone, which usually presents reducing conditions due to the high microbial activity which consumes the oxygen in the water. Within this zone there are important biochemical processes and redox reactions that affect groundwater quality.
The efficiency of RBF depends on local conditions including the hydrology and hydrogeology of the site, the geochemistry of water (from both the river and the aquifer), the geochemistry of microbial populations, and associated metabolic activity. This is the reason why is difficult to define general procedures for identifying appropriate sites to implement the RBF technique, as well as the expected efficiency of the process.
One limitation on the efficiency of RBF is the clogging of the bed and the banks of the river, which decreases the hydraulic conductivity in the hyporheic zone. This clogging can be caused by the infiltration of fine sediments, gas entrapment, biofilm formation related to microbiological activity, or the precipitation and co-precipitation of inorganic compounds, being the first of these the most influential factor in clogging formation.
Although the practice of riverbank filtration has been used in Europe for more than a century, the current understanding of the processes and mechanisms behind this technique are still very empirical. Besides, its use in tropical countries is almost nonexistent. At the PARH (Posgrado en Aprovechamiento de Recursos Hidraulicos) of the Universidad Nacional de Colombia in Medellin, we believe there is a great potential for RBF in our country, and that is why we are exploring this issue with more detail.
ACKNOWLEDGMENTS
This paper is the result of an extensive bibliographic review as part of the project “Integral management of joint use of surface and groundwater”, co-funded by COLCIENCIAS and the Universidad Nacional de Colombia. To both institutions the author wishes to express her most sincere gratitude. Much thanks also to her advisor, Professor Jaime Velez, PhD., (Universidad Nacional de Colombia) for his unconditional support.
REFERENCES
[1] Doussan, C., Ledoux, E. and Detay, M., River-groundwater exchanges, bank filtration, and groundwater quality: Ammonium Behavior. Journal of Environmental Quality, 27(6), pp. 1418-1427, 1998. [ Links ]
[2] Tufenkji, N., Ryan, J. N. and Elimelech, M., The promise of bank filtration. Environmental Science and Technology, 36 (21), pp. 422A-428A, 2002. [ Links ]
[3] Ray, C., Grischek, T., Schubert, J., Wang, J. Z. and Speth, T. F., A perspective of riverbank filtration. Journal of American Water Works Association (AWWA), 94 (4), pp. 149-160, 2002. [ Links ]
[4] Kuehn, W. and Mueller, U., Riverbank filtration: an overview. Journal of American Water Works Association (AWWA), 92 (12), pp. 60-69, 2000. [ Links ]
[5] Hiscock, K. M. and Grischek, T., Attenuation of groundwater pollution by bank filtration. Journal of Hydrology, 266 (3-4), pp. 139-144, 2002. [ Links ]
[6] Schubert, J., Hydraulic aspects of riverbank filtration - field studies. Journal of Hydrology, 266, pp. 145 - 161, 2002a. [ Links ]
[7] Sandhu, C., Grischek, T., Kumar, P. and Ray, C. Potential for riverbank filtration in India. Clean Techn Environ Policy, pp. 1-22 (DOI 10.1007/s10098-010-0298-0). 2010. [ Links ]
[8] Ray, C., Worldwide potential of riverbank filtration. Clean Technologies and Environmental Policy, 10, pp. 223-225, 2008. [ Links ]
[9] Grischek, T., Schoenheinz, D. and Ray, C., Siting and design issues for riverbank filtration schemes. In: Ray C, Melin G, Linsky RB (eds) Riverbank Filtration Improving Source-Water Quality. Kluwer Academic Publishers, Dordrecht, pp. 291-302, 2002. [ Links ]
[10] Gollnitz, W. D., Clancy, J. L., Mcwen, J. B. and Garner, S. C. Riverbank Filtration for IESWTR compliance. Journal of American Works Association (AWWA), 97 (12), 64-76. 2005. [ Links ]
[11] Jüttner, F. Elimination of terpenoid odorous compounds by slow sand and river bank filtration of the Ruhr River, Germany. Water Science and Technology, 31 (11), pp. 211-217, 1995. [ Links ]
[12] Jüttner, F. Efficacy of bank filtration for the removal of fragrance compounds and aromatic hydrocarbons. Water Science and Technology, 40 (6), pp. 123-128, 1999. [ Links ]
[13] Heberer, T., Massmann, G., Fanck, B., Taute, T. and Dünnbier, U., Behavior and redox sensitivity of antimicrobial residues during bank filtration. Chemosphere, 73, pp. 451-460, 2008. [ Links ]
[14] Schijven, J. F., Berger, P. and Miettinen, I., Removal of pathogens, surrogates, indicators, and toxins using Riverbank Filtration. In: Ray C, Melin G, Linsky RB (eds) Riverbank Filtration Improving Source-Water Quality. Kluwer Academic Publishers, Dordrecht, pp. 73-116, 2002. [ Links ]
[15] Yates, M. V., Gerba, C. P. and Kelley, L. M., Virus persistence in Groundwater. Applied and Environmental Microbiology, Vol. 49 (4), pp. 778-781, 1985. [ Links ]
[16] Gerba, C. P., Applied and theoretical aspects of virus adsorption to surfaces. Advances in Applied Microbiology, 30 pp. 133-168, 1984. [ Links ]
[17] De Roda Husman, A. M., Lodder, W. J., Rutjes, S. A., Schijven, J. F. and Teunis, P. F. M., Long-term inactivation study of three enteroviruses in artificial surface and groundwaters using PCR and cell culture. Applied and Environmental Microbiology, Vol. 75, (4), pp. 1050-1057, 2009. [ Links ]
[18] Aronino, R., Dlugy, C., Arkhangelsky, E., Shandalov, S., Oron, G., Brenner, A. and Gitis, V., Removal of viruses from surface water and secondary effluents by sand filtration. Water Research. 43, pp. 87-96, 2009. [ Links ]
[19] Schijven, J. F. and Hassanizadeh, S. M., Removal of viruses by soil passage: overview of modelling, processes, and parameters. Critical Reviews in Environmental Science and Technology, 30 (1), pp. 49-127, 2000. [ Links ]
[20] Reed, B. E., Matsumoto, M. R., Viadero, R. Jr. and Segar, R. L. Jr., Physicochemical processes. Water Environment Research, 71 (5), pp. 584-618, 1999. [ Links ]
[21] McDowell-Boyer, L. M., Hunt, J. R. and Sitar, N., Particle transport through porous media. Water Resources Research. 22, pp. 1901-1921. 1986. [ Links ]
[22] Wang, J., Riverbank filtration case study at Louisville, Kentucky. In: Ray C, Melin G, Linsky RB (eds) Riverbank Filtration Improving Source-Water Quality. Kluwer Academic Publishers, Dordrecht, pp. 117-145, 2002. [ Links ]
[23] Weiss, W. J., Bouwer, E. J., Ball, W. P., O'Melia, C. R., Arora, H. and Speth, T. F., Reduction in disinfection byproduct precursors and pathogens during riverbank filtration at three Midwestern United States drinking-water utilities. In: Ray C, Melin G, Linsky RB (eds) Riverbank Filtration Improving Source-Water Quality. Kluwer Academic Publishers, Dordrecht, pp. 147-173, 2002a. [ Links ]
[24] Gollnitz, W. D., Clancy, J. L., Whitteberry, B. L. and Vogt, J. A. RBF as a microbial treatment process. Journal of American Water Works Association (AWWA), 95 (12), pp. 56-66, 2003. [ Links ]
[25] Schubert, J., Water-quality improvements with riverbank filtration at Düsseldorf waterworks in Germany. In: Ray C, Melin G, Linsky RB (eds) Riverbank Filtration Improving Source-Water Quality. Kluwer Academic Publishers, Dordrecht, pp. 267 - 277, 2002b. [ Links ]
[26] Schubert, J., German experience with riverbank filtration systems. In: Ray C, Melin G, Linsky R. (eds) Riverbank Filtration Improving Source-Water Quality. Kluwer Academic Publishers, Dordrecht, pp. 35 - 48, 2002c. [ Links ]
[27] Gibert, J., Fournier, F. and Mathieu, J., The groundwater/surface water ecotone perspective: state of the art. In: Gibert J, Mathieu J, Fournier F (eds) Groundwater/Surface Water Ecotones: Biological and Hydrological Interactions and Management Options. Cambridge University Press: New York, pp. 3-6, 1997. [ Links ]
[28] Bourg, A. C. and Bertin, C., Biogeochemical processes during the infiltration of river water into an alluvial aquifer. Environmental Science and Technology, 27, pp. 661-666, 1993. [ Links ]
[29] Ndabigengesere, A. and Narasiah, K. S., Quality of water treated by coagulation using Moringa oleifera seeds. Water Resources, 32 (3), pp. 781-791, 1998. [ Links ]
[30] Mucha, I., Banský, L., Hlavatý, Z. and Rodák, D., Impact of riverbed clogging - colmatation - on ground water. In: Hubbs SA (ed) Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Springer, Dordrecht, pp. 43-72, 2006. [ Links ]
[31] Kim, S. B. and Corapcioglu, M. Y., Contaminant transport in riverbank filtration in the presence of dissolved organic matter and bacteria: a kinetic approach. Journal of Hydrology, pp. 266, 269-283, 2002. [ Links ]
[32] Kim, S. B., Corapcioglu, M. Y. and Kim, D. J., Effect of dissolved organic matter and bacteria on contaminant transport in riverbank filtration. Journal of Contaminant Hydrology, 66, pp. 1-23, 2003. [ Links ]
[33] Richardson, S. D. Disinfection by-products and other emerging contaminants in drinking water. TrAC Trends in Analytical Chemistry, 22 (10), pp. 666-684, 2003. [ Links ]
[34] Weiss, W. J., Bouwer, E. J., Ball, W. P., O'Melia, C. R., Lechevallier, M. W., Arora, H. and Speth, T. F., Riverbank filtration - fate of DBP precursors and selected microorganisms. Journal of American Water Works Association (AWWA), 95 (10), pp. 68-81. 2003b. [ Links ]
[35] Vanek, V., Heterogeneity of groundwater-surface water ecotones. In: Gibert J, Mathieu J, Fournier F (eds) Groundwater/Surface Water Ecotones: Biological and Hydrological Interactions and Management Options. Cambridge University Press: New York; pp 151-161, 1997. [ Links ]
[36] Schubert, J., How does it work? Field studies on riverbank filtration. In: Julich W, Schubert J (eds) Proceedings of the International Riverbank Filtration Conference. IAWR, Dusseldorf, Germany, pp. 41 - 55. 2000. [ Links ]
[37] Gollnitz, W. D., Whitteberry, B. L. and Vogt, J. A., Riverbank filtration: induced infiltration and groundwater quality. Journal of American Works Association (AWWA), 96 (12), pp. 98-110. 2004. [ Links ]
[38] Zhou, N., Matsumoto, T., Hosokawa, T. and Suekane, T., Pore-scale visualization of gas trapping in porous media by X-Ray CT Scanning. Flow Measurement and Instrumentation, 21 (3), pp. 262-267, 2010. [ Links ]
[39] Baveye, P., Vandevivere, P., Hoyle, B. L., Deleo, P. C. and De Lozada, D. S., Environmental impact and mechanisms of the biological clogging of saturated soils and aquifer materials. Critical Reviews in Environmental Science and Technology, 28 (2), pp. 123-191. 1998. [ Links ]
[40] Schubert, J., Sifgnificane of hydrologic aspects on RBF performance. In: Hubbs SA (ed) Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Springer, Dordrecht, pp. 1 - 20, 2006a. [ Links ]
[41] Schubert, J., Experience with riverbed clogging along the Rhine River. In: Hubbs SA (ed) Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Springer, Dordrecht, pp. 221 - 242, 2006b. [ Links ]
[42] Hubbs, S. A., Evaluating streambed forces impacting the capacity of riverbed filtration systems. In: Hubbs SA (ed) Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Springer, Dordrecht, pp. 21-42, 2006a. [ Links ]
[43] Schafer, F., Use of aquifer testing and groundwater modeling to evaluate aquifer/river hydraulics at Louisville Water Company, Luisville, Kentucky, USA. In: Hubbs SA (ed) Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Springer, Dordrecht, pp. 179-198, 2006. [ Links ]
[44] Hubbs, S. A., Changes in riverbed hydraulic conductivity and specific capacity at Louisville. In: Hubbs SA (ed) Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Springer, Dordrecht, pp. 199-220, 2006b. [ Links ]
[45] Engesgaard, P., Seifert, D. and Herrera, P., Bioclogging in porous media: tracer studies. In: Hubbs SA (eds) Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Springer, Dordrecht, pp. 93-118, 2006. [ Links ]
[46] Seifert, D. and Engesgaard, P., Use of tracer tests to investigate changes in flow and transport properties due to bioclogging in porous media. Journal of Contaminant Hydrology, 93, pp. 58 - 71, 2007. [ Links ]
[47] Sutherland, I. W. Biofilms exopolysaccharides: a strong and sticky framework. Microbioloty, 147, pp. 3 - 9. 2001. [ Links ]
[48] Caldwell, T. G., Presentation of data for factors significant to yield from several riverbank filtration systems in the U.S. and Europe. In: Hubbs SA (ed) Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Springer, Dordrecht, pp. 299-344, 2006. [ Links ]
[49] Stuyfzand, P. J., Juhasz-Holterman, M. H. A. and De Lange, W. J., Riverbad filtration in the Netherlands: well fields, clogging and geochemical reactions. In: Hubbs SA (ed) Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Springer, Dordrecht, pp. 119 - 153, 2006. [ Links ]














