Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.79 no.174 Medellín July/Aug. 2012
CONTINUOUS PRODUCTION OF ETHANOL IN PACKED BED-BIOREACTORS WITH IMMOBILIZED YEAST CELLS ON LIGNOCELLULOSIC WASTE
PRODUCCIÓN CONTINUA DE ETANOL EN BIORREACTORES DE LECHO EMPACADO CON CÉLULAS DE LEVADURA INMOVILIZADAS EN RESIDUOS LIGNOCELULÓSICOS
LINA MARÍA AGUDELO ESCOBAR
Ph.D. (c). Profesora Asistente, Universidad de Antioquia, Medellín, Colombia, agudelo.linamaria@gmail.com
URIEL SALAZAR ÁLVAREZ
Ingeniero Químico, Universidad de Antioquia, Medellín, Colombia
MARIANA PEÑUELA
Ph.D. Profesora Asistente, Universidad de Antioquia, Sede de Investigación Universitaria (SIU)
Received for review November 23th, 2011, accepted May 16th, 2012, final version May, 31th, 2012
ABSTRACT: Continuous processes with immobilized cells are a good alternative for improving the efficiency and the performance of alcoholic fermentations. The potential use of raw materials obtained from agro-industrial waste as supports for cell immobilization was recently evaluated. In this work, we evaluated the continuous production of ethanol in packed-bed reactors with yeast cells immobilized on wood shaving, cane bagasse, corn leave, and corn cob lignocellulosic waste. We used glucose as a carbon source to establish the reference conditions and we made the fermentations with commercial sucrose. We also evaluated the glucose syrup obtained from cassava flour as alternative substrate. The experiments were performed on a laboratory level in column reactors of 250 mL. The cane bagasse was the material on which the highest amount of cells was immobilized. A value of 0.047 g Biomass/g Carrier (gX/gC) was obtained. For the fermentation performed with glucose, the productivity reached was 13.33 ± 1.5 g/L h. A similar value, 13.00 ± 0.02 g/L h was reached for the productivity of fermentation with sucrose. In the fermentations performed with glucose syrup, a higher amount of ethanol was obtained in the process that used corn cob as the carrier. 31.99 ± 1.93 g/L of ethanol was produced and the productivity of 10.66 ± 0.64 g/L h was reached. The results obtained have allowed us to establish the potential this lignocellulosic waste has as carriers for cell immobilization for the continuous production of ethanol, and the possibility of using glucose syrup as an alternative substrate.
KEYWORDS: Biofuels, Ethanol, Continuous process, Yeast immobilization, Lignocellulosic waste, Alcoholic fermentation, Yeasts
RESUMEN: Los procesos continuos con células inmovilizadas son una alternativa para mejorar la eficiencia y el rendimiento de las fermentaciones alcohólicas. Recientemente, se han comenzado a evaluar materias primas derivadas de residuos agroindustriales y su aplicación como soportes promisorios para la inmovilización celular. En este trabajo se evaluó la producción continua de etanol en reactores de lecho empacado con células de levadura inmovilizadas en residuos lignocelulósicos de viruta, bagazo de caña, capacho y tusa de maíz. Se empleó glucosa como fuente de carbono para establecer las condiciones de referencia y se realizaron las fermentaciones con sacarosa comercial. Además, se evaluó como sustrato alternativo el jarabe glucosado obtenido de la harina de yuca. Las experimentaciones fueron realizadas a nivel de laboratorio en reactores de columna de 250 mL. El bagazo de caña fue el material en el que se inmovilizó mayor cantidad de células. Se obtuvo un valor de 0,047 g de Biomasa/g de soporte (gX/gC). Para la fermentación realizada con glucosa, la productividad alcanzada fue de 13,33 ± 1,5 g/L h. Un valor similar, 13,00 ± 0,02 g/L h fue alcanzado para la productividad de la fermentación con sacarosa. En las fermentaciones realizadas con jarabe glucosado, se obtuvo mayor cantidad de etanol en el proceso que empleó tusa como soporte. Se produjeron 31,99 ± 1,93 g/L de etanol y se alcanzó una productividad de 10,66 ± 0,64 g/L h. Los resultados obtenidos han permitido establecer el potencial que tienen estos residuos lignocelulósicos como soportes en la inmovilización celular para la producción en continuo de etanol, y la posibilidad de emplear el jarabe glucosado como sustrato alternativo.
PALABRAS CLAVE: Biocombustibles, Etanol, Proceso continuo, Células inmovilizadas, Residuos lignocelulósicos, Fermentaciones alcohólicas, Levaduras
1. INTRODUCTION
One of the more relevant biofuels for the substitution of fossil fuels is ethanol [1], which is mainly produced by alcoholic fermentation in batch processes. However, the performance and costs of production of this technology are still high compared to fossil fuels [2]. Many possibilities of optimization have been suggested for obtaining this biofuel, and one of them has to do with the usage of more efficient production technologies. The continuous processes are an alternative for improving the efficiency and performance of alcoholic fermentation and for reducing the costs of production. The continuous processes that use immobilized cells show several advantages with respect to continuous processes with free cells [3,18,20]. The immobilization matrixes more frequently used have been polymeric gels like alginate, where the cells are immobilized for entrapment into the gel. However, this method of immobilization is not too viable on an industrial scale, due to the high cost of raw material and the relative complexity of preparation of the biocatalysts (carrier-cell) with respect to the operative lifetime of the process [4,21,14]. The potential use of raw materials obtained from agro-industrial waste as supports for cell immobilization was recently evaluated.[15–17] On these carriers, the cells are immobilized by adsorption, a simple and cheap technique [5,7,13].
In this work, we evaluated the continuous production of ethanol in packed-bed reactors with yeast cells immobilized on wood shaving, cane bagasse, corn leave, and corn cob lignocellulosic waste. We used glucose as a carbon source to establish the reference conditions and we made the fermentations with commercial sucrose. We also evaluated the glucose syrup obtained from cassava flour as an alternative substrate.The experiments were performed on a laboratory level in column reactors of 250 mL.
2. MATERIALS AND METHODS
2.1. Microorganisms and Medium Fermentation
We used the commercial yeast Saccharomyces cerevisiae ETHANOL RED, mantained at a temperature of 4 °C. The fermenting medium used for the experiments was the following: (concentration per litre) 100 g of reductive sugar, 3.6 g of peptone, 4 g of yeast extract, 2 g of KH2PO4, 1 g of MgSO4.7H2O, and 3 g of (NH4)2SO4. The pH was set to 5.0 before sterilization at 15 psi during 15 min.
2.2. Carriers for immobilization
The lignocellulosic waste used were wood shaving, cane bagasse, corn leave, and corn cob, which were obtained from some agro industries in the region. The materials were previously prepared for the experiments by applying washing processes with boiling water, drying up at 105 °C, and with a mechanical reduction of size by using a blade mill. We obtained pieces of material of sizes between 3.4 to 10 mm.
2.3. Immobilization process
The cellular immobilization was carried out in a column reactor of 250 mL, provided with a temperature control jacket. The material was packed up to 70% of the volume of the reactor, and before the immobilization, it was sterilized for 20 min at 15 psi and 120 °C. The immobilization was performed by recirculating cell suspension with a concentration of 15 g/L of biomass. A Cole Parmer peristaltic pump 7518-10 (model 77202-60) was used, operating at a flow of 0.33 mL/min. The process of immobilization was carried out for 12 h.
2.4. Batch fermentation
Once the immobilization process in the column bioreactor was finished, we began the batch fermentation by pumping the medium fermentation until the reactor filled up. The fermentation time of 12 h. was established considering the results of kinetics obtained in previous works [3,6]. The control of the temperature in the process was performed by permanent recirculation of water at 30 °C through the jacket of the reactor. We took the sample at the end of the fermentation.
2.5. Continuous fermentation
For continuous fermentation, a feeding flow of 1.4 mL/min was used. The established time of residence (t) was 3 h, and the reactor was operated this way for 6 times of residence (18 h). Before starting the continuous process, we carried out the stages of cell immobilization and batch fermentation previously described. The control of the temperature in the process was performed by permanent recirculation of water at 30 °C through the jacket of the reactor. Stable system operation was achieved after the fourth residence time, when the sugar concentration in the samples was similar. The samples were taken at the output of the reactor, once the system was in a stable state.
2.6. Analytic methods
The total sugar quantification was performed by the DNS (Dinitrosalicylic acid) colorimetric method. The absorbance reading was done with a spectrophotometer (Spectronic Genesis 2, model 336009, serial no. LR 45227) at 540 nm [8]. The concentration of biomass was determined by optic density at 600 nm using a curve of dry weight. The concentration of alcohol was determined by HPLC. We used a C-610H column with an internal diameter of 7.8 mm and 30 cm long. The flow of the mobile phase was 0.5 mL/min. The detection by UV absorbance was set to 210 nm. The temperature was set to 30 °C. The amount of immobilized biomass was established by the modified dry-weight technique [9]. In order to confirm immobilization and determine the differences between the carriers, we obtained images by scanning electronic microscopy (SEM).
3. RESULTS AND DISCUSSION
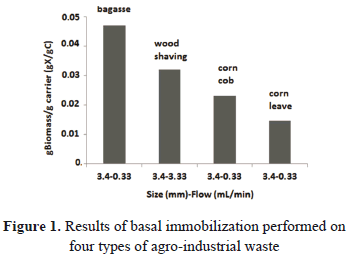
Figure 1 shows the results obtained in the cell immobilization performed on the 4 carriers evaluated. It is observed that the cane bagasse was the material in which the highest amount of cells was immobilized, 0.047 gX/gC. The process of immobilization was carried out with a flow of 0.33 mL/min and the size of the carrier used was an average of 3.4 mm. For the wood shaving and corn cob, we obtained an amount of immobilized biomass of 0.032 and 0.023 gX/gC, respectively. In the case of the corn cob, the immobilization was obtained under the same conditions; that is, a flow of 0.33 mL/min and a 3.4 mm size of the carrier. For the wood shaving, the amount of immobilized biomass was obtained with a flow of 3.33 mL/min and a 3.4 mm size of the carrier. The corn leave carrier was the material in which the lowest amount of cells was immobilized. The value obtained was 0.0147 gX/gC, using a flow of 0.33 mL/min, and a 3.4 mm size for the carrier.
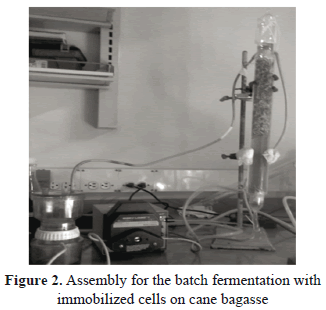
The fermentations with glucose as the carbon source were performed using cane bagasse as the carrier. In Fig. 2, we present the experimental set-up made for these fermentations. The reactor was fed with fermentation medium throughout the peristaltic pump. The filter attached to the reactor output was used to retain the cells that were not immobilized within the process.
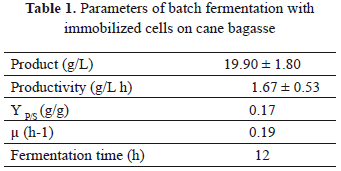
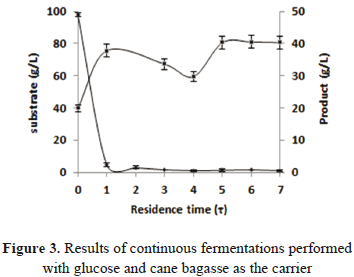
The kinectics of the immobilized cells was determined during 12 h in a batch fermentation with an initial amount of cells of 0.046 gX/gC. In this system, the substrate consumption and generation of the product was given only by the immobilized cells thanks to the filtering equipment installed at the reactor output. This allowed for the constant removal of the free cells that grew up and/or were un-immobilized in the packed bed of the reactor. The final concentration of ethanol obtained was 19.90 g/L, reaching a productivity of 1.670 g/L h and an amount of immobilized biomass of 0.048 gX/gC at the end of the process. The yield of ethanol production was some 38% of the theoretical value. We obtained a consumption of 96.42% of substrate after 12 h of fermentation. The parameters obtained in the batch process are shown in Table 1.
For the continuous process, we used a time of residence of 3 h and a flow of 1.4 mL/min. The samples were taken during 6t equivalent to 18 h. Prior to the start of the continuous process, we performed a batch stage of 12 h. In Fig. 3, we can observe the results for the continuous fermentations. We noticed that the system stabilizes when it passes the fourth time of residence (4t). The consumption of glucose was high when it passed the first time of residence (1t). The maximum concentration of glucose obtained at the output of the reactor was 5 g/L. However, upon reaching stable operation, the value was 1.54 g/L.
The production of ethanol obtained at the output of the reactor was 19.9 g/L at the end of the batch stage. Afterwards, it got stabilized on the fifth time of residence with a value of 40 g/L. Compared to the theoretical yield, the performance reached in the experiment was 78%, which indicates a high rate of conversion from substrate to product. The productivity reached was 13.33 ± 1.5 g/L h, obtained with the same amount of immobilized biomass than in the batch process.
Table 2 shows the parameters obtained in the continuous fermentation. The productivity reached in this process is some 86% better than the batch fermentations. The results obtained in the continuous fermentations are significant if compared to the results obtained by other researchers [12]. For instance, the productivity we reached is 21% better than the value reported by Vargas, et al., 2001 [3], who reached a productivity of 9.42 g/L h in a continuous fermentation with cells immobilized in calcium alginate, carried out under the same operational conditions of flow rate, time of residence, and temperature. Additionally, we reached a better productivity than Plessas S., et al., 2006 [10,11], who reported a productivity of 128.3 g/L per day corresponding to 5.35 g/L h in a continuous process with cells immobilized on orange peels.
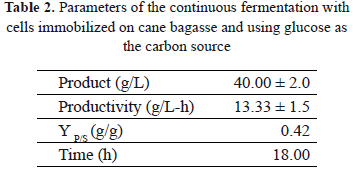
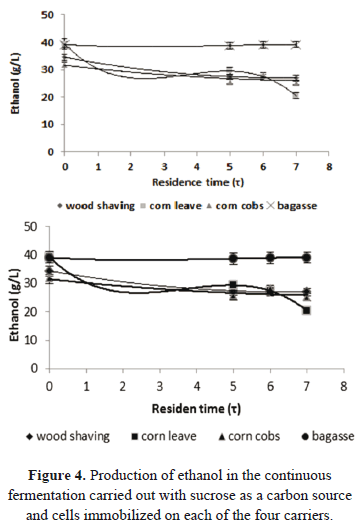
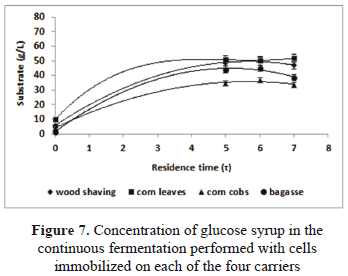
We made continuous fermentation using the biocatalysts obtained with the four materials. As a carbon source, we evaluated sucrose and glucose syrup obtained from cassava flour. Figures 4 and 5 show the results obtained for the continuous fermentation carried out with sucrose. And Figs. 6 and 7 show the results for the fermentations with glucose syrup.
As observed in Fig. 4, the stable operational state of the reactor was reached after the fourth time of residence (4t). The highest concentration of ethanol was reached in the fermentation that used the cane bagasse as a carrier. Although the sugars are not completely consumed at the end of the process, the production of ethanol was sustained in a very similar value than the one obtained with glucose, reaching a productivity of 13.00 ± 0.02 g/L h, the same value reached in the fermentation with glucose.
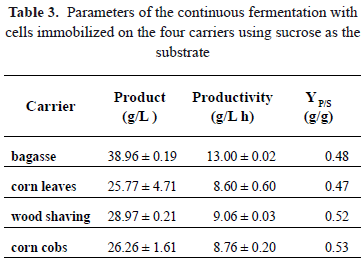
Table 3 shows the values of productivity reached with the other materials. The productivities reached in fermentations with cells immobilized on corn leaves, corn cob, and wood shaving are similar to each other. The consumption of sucrose in the fermentations is different to the consumption of glucose (Fig. 5). This indicates that there is a change in the kinetics of the organism, which becomes evident in the increase of the yield of a substrate into the product of the fermentation (YP/S). The maximum theoretical yield of sucrose to ethanol is 0.54 g ethanol/g sucrose. The values obtained in the fermentations with this substrate indicate conversions of 89 and 87% for bagasse and corn leaves, respectively. In the case of fermentations conducted with wood shaving and corn cob as carriers, conversions relative to the theoretical value were, respectively, 96 and 98%. Differences in yield may result from variations in the microenvironmental conditions generated within the bioreactor, probably caused by the particular characteristics of each carrier [19].
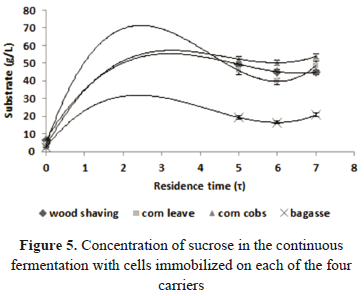
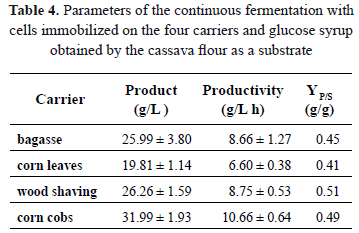
The results of the fermentations carried out with glucose syrup obtained from cassava flour are shown in Figs. 6 and 7. The fermentation which produced the highest amount of ethanol was the one using corn cobs as a carrier. We produced 31.99 ± 1.93 g/L and we reached a productivity of 10.66 ± 0.64 g/L h. These results are very relevant if compared with the values obtained in the traditional batch fermentations as well as the results obtained by other researches, who used different materials in the immobilization of cells for the production of ethanol [11–20].
As observed in Fig. 7, the glucose syrup was not fully consumed. At the output of the reactor we obtained concentrations from 51.15 ± 1.02 for the corn leaves to 35.03 ± 1.70 for the corn cobs. These results are similar to the values obtained with the sucrose.
Table 4 shows the parameters obtained in the continuous fermentation with glucose syrup as a carbon source. The substrate yields into the product (YP/S) were lower than the values obtained in the fermentation with sucrose. However, these values are higher than the ones obtained in the fermentation with glucose. This is possibly due to the fact that glucose syrup contains other fermentable sugars such as maltose. This indicates the potential that glucose syrup has as an alternative substrate for ethanol production.
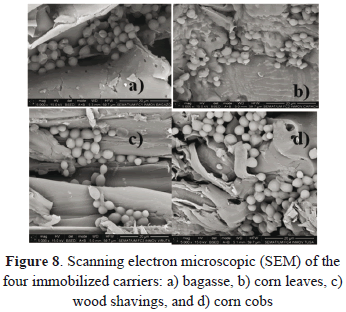
Figure 8 shows the cells immobilized on each of the four evaluated carriers. Initially, the cells have more affinity for folds and rough surfaces. However, as the contact of the cells with the carrier increases, and the fermentation time passes, we expected the immobilization to occur on the whole surface of the carrier. We could appreciate some differences between the surfaces of the four carriers. The features of the surfaces may change the process of cells immobilization as well as the yield in the production of ethanol.
4. CONCLUSIONS
The results obtained have allowed us to establish the potential these lignocellulosic wastes have as carriers in the immobilization of cells for the continuous production of ethanol. We have also established that the glucose syrup obtained from the enzymatic hydrolysis of cassava flour can be used as an alternative substrate in alcoholic fermentations. We have obtained better yields than the batch processes, currently being used on an industrial level. Additionally, we have obtained better yields than continuous alcoholic fermentations performed with free cells and fermentations performed with cells immobilized on other materials. However, an industrial implementation of this process requires further optimizations that allow for one to increase the amount of cells immobilized on the carriers, as well as improvements in the operational conditions of the process. In this way, the sugars provided in the reactor input could be fully consumed, increasing the yield and the productivity even more.
ACKNOWLEDGEMENTS
We kindly thank the Biotechnology and Biotransformation Groups of the Universidad de Antioquia for their support. It has greatly contributed to the development of this research.
REFERENCES
[1] Monsalve, J. F., Medina De Perez, V. I. y Ruiz, A. A., Producción de etanol a partir de la cáscara de Banano y de almidón de yuca. Dyna, 73 (150), 21-27, 2006. [ Links ]
[2] Nakari-Setälä, T., Azeredo, J., Henriques, M., Oliveira, R., Teixeira, J., Linder, M. and Penttila, M., Expression of a Fungal Hydrophobin in the Saccharomyces cerevisiae Cell Wall: Effect on cell Surface Properties and Immobilization. Applied and environmental microbiology, 68(7), pp. 3385-3391, 2002. [ Links ]
[3] Vargas, G., Peñuela, M., Echeverri, M., Ortiz, M.E, Escobar, M. C. y Quintero, J. C., Producción de alcohol por fermentación con levaduras libres e inmovilizadas. Revista Facultad de Ingeniería Universidad de Antioquia, 22, pp. 91-97, 2001. [ Links ]
[4] Bekatorou, A. and Kanellaki, M., Fermentation technology: immobilized cells & low temperature processes. SciTopics. Retrieved August 22, 2011, Available:http://www.scitopics.com/Fermentation_technology_Immobilized_cells_low_temperature_processes.html [31 March ,2010] [ Links ].
[5] Domingues, L., Dantas, M. M., Lima, N., and Teixeira, J.A., Continuous ethanol fermentation of lactose by a recombinant flocculating Saccharomyces cerevisiae strain. Biotechnology and Bioengineering 64, pp. 692– 697, 1999. [ Links ]
[6] Agudelo, E. L. M., Escalado de un biorreactor con células inmovilizadas para la producción de etanol. [Msc research investigation], Universidad de Antioquia, 2007. [ Links ]
[7] Brányik, T., Vicente, A. A., Machado, C. J. M. and Teixeira, J.A., Continuous primary beer fermentation with brewing yeast immobilized on spent grains. Journal Institute of Brewing 108, pp. 410– 415, 2002. [ Links ]
[8] Iconomopoulou, M., Kanellaki, M., Psarianos, K. and Koutinas, A. A., Delignified cellulosic material supported biocatalyst as freeze-dried product in alcoholic fermentation, Journal Of Agriculture Food Chemistry, 48, 958-96, 2000. [ Links ]
[9] Brányik, T., Vicente, A.A., Machado, C.J.M. and Teixeira, J.A., Spent grains- a new support for brewing yeast immobilization, Biotechnology Letters, 23, pp. 1073-1078, 2001. [ Links ]
[10] Bardi, E.P. and Koutinas, A.A., Immobilization of yeast on delignified cellulosic material for room temperature and low-temperature wine making, Journal Agriculture Food Chemistry, 42, pp. 221-226, 1994. [ Links ]
[11] Plessas, S., Bekatorou, A., Kanellaki, M., Psarianos, C. and Koutinas, A. A., Cells immobilized in a starch-gluten-milk matrix usable for food production, Food Chemistry, 89, 175-179, 2005. [ Links ]
[12] Brányik, T., Vicente, A. A., Dostálek, P. and Teixeira, J. A., Continuous beer fermentation using immobilized yeast cell bioreactor systems. Biotechnology Progress. 21, pp. 653-663, 2005. [ Links ]
[13] Mallouchos, A., Reppa, P., Aggelis, G., Koutinas, A., Kanellaki, A. and Komaitis, M., Grape skins as a natural support for yeast immobilization. Biotechnology. Letter,24, pp. 1331–1335, 2002. [ Links ]
[14] Vasconcelos, J. N., Lopes, C. E. and França, F. P., Continuous ethanol production using yeast immobilized on sugar-cane stalks. Brazilian Journal of Chemical Engineering, 21(03), pp. 357 – 365, 2004. [ Links ]
[15] Baptista, C.M.S.G., Cóias, J.M.A., Oliveira, A.C.M., Oliveira, N.M.C., Rocha, et. al. Natural immobilisation of microorganisms for continuous etanol production, Enzyme and Microbial technology, 40, pp. 127-131, 2006. [ Links ]
[16]Millar, G. L., Use of DNS for determination of reducing sugar. Analytical Chemistry, 31(3), pp. 426-428, 1959. [ Links ]
[17] Agudelo, E. L. M., Peñuela, M. y Quintero, J., Proceso de inmovilización celular en materiales lignocelulósicos para la producción de etanol como combustible, Memorias del Congreso de Ingeniería Química, Medellín, Colombia, pp. 52-56, octubre 2009. [ Links ]
[18] Dragone, G., Mussatto, S.I., Almeida, E. y Silva, J. B. Levaduras inmovilizadas en soporte lignocelulósicos para la producción de cerveza, Brazilian Journal of Food Technology, 5° SIPAL, 70-73, 2005. [ Links ]
[19] Plessas, S., Kourkoutas, Y., Psarianos, C., Kanellaki, M. and Koutinas, A. A., Continuous bakers yeast production using orange peel as promoting support in the bioreactor, Journal of the Science of Food and Agriculture, 86(3), pp. 407–414, 2006. [ Links ]
[20] Plessas, S., Bekatorou, A., Koutinas, A. A., Soupioni, M., Banat Im, Ymarchant, R., Use of Saccharomyces cerevisiae cells immobilized on orange peel as biocatalyst for alcoholic fermentation. Bioresources Technology, 98(4), .860–5, 2007. [ Links ]
[21] Brányik, T., Vicente, A., Oliveira, R. and Teixeira, J., Physicochemical surface properties of brewing yeast influencing their immobilization onto spent grains in a continuous reactor, Biotechnology and Bioengineering, 88(1), pp. 84-93, 2004. [ Links ]