Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.79 no.176 Medellín nov./dez. 2012
OSTEOBLAST ADHESION ON SCAFFOLDS OF PLA-PLG-HYDROXYAPATITE-CHITOSAN-ZINC BY ELECTROACTIVATION
ADHESIÓN DE OSTEOBLASTOS SOBRE ANDAMIOS DE PLA-PLG-HIDROXIAPATITA-QUITOSANO POR ELECTROACTIVACIÓN
HUGO ARMANDO ESTUPIÑAN DURAN
PhD, Departamento de Materiales y Minerales,Universidad Nacional de Colombia, profesor asistente, haestupinand@unal.edu.co
DARIO YESID PEÑA BALLESTEROS
PhD, Escuela de Ingeniería Metalúrgica y Ciencia de Materiales, Universidad Industrial de Santander, Profesor asociado, dypena@uis.edu.co
CUSTODIO VÁSQUEZ QUINTERO
MSc, Escuela de Ingeniería Metalúrgica y Ciencia de Materiales,Universidad Industrial de Santander, Profesor Titular, custodio@uis.edu.co
Received for review January 26th, 2012, accepted July 27th, 2012, final version August, 27th, 2012
ABSTRACT: This paper presents results of the study of the phenomenon of electroactivation of system cell- polylactic acid (PLA) and polyglycolic acid (PLG) with additions of bioactive elements of chitosan (q), bioceramic (bc), and zinc. The main purpose is to explain the increase in the adhesion of cells as a result of the change in the hydrophilicity of a cell regeneration matrix and the variation in intracellular ion exchange, induced by the application of electric overpotential and bioactive element additions to the polymers.
KEYWORDS: electroactivation, scaffold, impedance, bioactivity
RESUMEN: En este trabajo se presentan los resultados del estudio del fenómeno de la electroactivación del sistema de células - ácido poliláctico (PLA) - ácido poliglicólico (PLG) con adiciones de elementos bioactivos de quitosano (q), biocerámico (bc), y zinc. El objetivo principal es explicar el incremento en la adhesión de células como un resultado del cambio en la hidrofilicidad de la matriz de regeneración celular y la variación en el intercambio iónico intracelular, inducida por la aplicación de sobrepotenciales eléctricos y las adiciones de elementos bioactivos a los polímeros.
PALABRAS CLAVE: electroactivación, andamio, impedancia, bioactividad
1. INTRODUCTION
Polymers are widely accepted materials in tissue engineering. The role of polymers in the science of biomaterials is to direct cell growth, either from cells from adjacent tissues or cells seeded on the materials prior to implantation. Therefore, the polymer must provide adequate adhesion, promote cell proliferation and differentiation, and in certain cases, promote cell migration [1,2].
In a scaffold of bone cellular regeneration composed for a bioceramic hydroxyapatite (HAP) and a matrix polymer of polylactic acid (PLA), we have studied the mechanical properties, the degradation rate, and biocompatibility. It has been shown that the presence of HAP in the PLA determines an increase in cell proliferation [1-3]. Despite its degradability, the PLA can function as polyelectrolytes with small additions of zinc. This material mixed with polyglycolic acid, a bioceramic (e.g., HAP), and chitosan, can allow surface functionalization to promote cell adhesion increase [3-5]. Chitosan as a component of a scaffold influences the stabilization of the structure between the elements apatitic bioactive polymers and cells. Moreover, cellular signaling is promoted by its similarity to the proteoglycans and glycosaminoglycans present in the natural ECM. This also favors the reaction of hydroxyl on the surface material, promoting the local pH increase to levels that ensure the survival of cells and their growth processes [6,7].
The cells have an intracellular space between lipid membrane walls through which vascular vessels are located that allow for ion exchange. Previous research has shown that cells respond to short duration electrical stimuli, causing physical and chemical changes that increase ionic conductivity, the ability to associate with other cells, and the ability to form dipole-dipole bonds with biomaterial surfaces that have the opposite electrical charge of the cells. It is known that the polarity of cell membranes is clearly negative, with a greater affinity for positively-charged materials. Consistent with the electrical property of cells, bone has piezoelectric properties, so bone cells respond electrically to mechanical disturbance or a mechanical response from electrical stimulation [8-12].
It has been shown that interfering with the hydroxylation reaction and the phosphorylation of the cell membrane increases the ion exchange di-calcium into the cell. In parallel fashion, the bioceramic resorption of calcium promotes the exchange of ions through the transmembrane cell and into physiological fluid [13-15].
This work shows the results of a study of the phenomenon of the electroactivation system cell/scaffold of polylactic acid (PLA) and polyglycolic acid (PLG) with additions of bioactive elements of chitosan, bioceramic (HAP), and zinc. The main purpose was to improve intracellular ion exchange and explain the increase in cell adhesion and protein resulting from the change in the hydrophilicity of the scaffold induced by applying an electric overpotential and the additions of bioactive elements.
2. MATERIALS AND METHODS
2.1 Synthesis of materials
To obtain the samples of the scaffold, a co-polymerization was made using 1% Zn as a catalyst with 40 h of polycondensation for the PLA and 0.2% tin chloride with 20 h of polycondensation for PLG. The synthesis of the PLGA copolymer was performed using the evaporation/solvent extraction method, using chloroform as a solvent.
The composition of the scaffold was 70:30 (PLA:PGA). This was mixed with hydroxyapatite at 10% w/v, using chloroform as the solvent, which was removed to T = 150 ºC, approximately. For the transformation of the copolymer (PLA-PGA) in a polyelectrolyte, excess zinc salt was added as a doping agent to modify the conductivity of the scaffold at concentrations between 0.5 and 2% w/v.
2.2. Description of techniques for assessing cell adhesion
The electrical properties of the cell-scaffold/physiological medium (RPMI) heterogeneous system, were evaluated by using the EIS technique. Equivalent circuit models proposed in the software ZVIEW allowed for us to obtain information on the following interfaces: RPMI/HOS osteoblast cells, HOS osteoblastic cells/scaffold, and scaffold/quartz crystal. Optical microscopy and SEM were used to assess cell adhesion.
Three samples of scaffold were obtained as coatings on quartz crystals from solutions of PLA-PLG dissolved in acetone 15% w/v, HAP 10% w/v chitosan at 0, 3, and 7% w/v during a time of 5 min and 3 volts, a set-up of a voltage source where the cathode was the quartz crystal Au-Cr and an austenitic steel sheet as the anode. Subsequently, the crystal was irradiated with UV light. For electrochemical measurements and QCM, we used a vertical flat cell, where the working electrode was a coated quartz crystal, platinum counter electrode, and reference electrode Ag-AgCl. The electrochemical measurements were performed using a potentiostat Gamry 600. The impedance spectroscopy measurements were performed in a frequency range of 0.01 to 100,000 Hz. The amplitude of the AC signal was 10mV. EIS measurements were performed under a stimulation potential of 0 V, 50 mV, and 50 mV. This over potential was obtained by the technique of cyclic voltammetry.
2.3. Cell culture
The HOS cell line obtained from American Type Culture Collection (ATCC), consisting of osteoblastic cells from a human osteosarcoma has been used.
A cell culture was carried out in Falcon® culture flasks under sterile conditions at 37 °C ± 0.5 °C and 5% CO2 in RPMI 1640 medium with 1% L-glutamine, 1% antibiotics (penicillin/streptomycin) and supplemented with 10% FBS (fetal bovine serum) without phenol red, pH = 7.2. The culture medium was changed every 2 days to ensure an adequate supply of nutrients in the culture dish and/or electrochemical cell. Fetal bovine serum was purchased from Hyclone Laboratories. A concentration of osteoblasts between 15,000 and 30,000 cells per milliliter of culture medium was used for adhesion tests.
3. RESULTS AND DISCUSSION
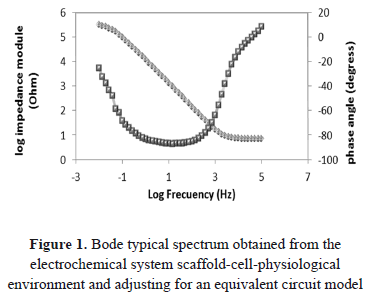
The Bode and Nyquist spectra obtained by electrochemical tests HOS cell adhesion on scaffolds of PLA-PLG-chitosan-hydroxyapatite-zinc, were fitted to equivalent circuit models using the proposed Zview 32 software. The error obtained from these adjustments in terms of chi2 was less than 10-4 between the experimental data and simulated data, which was considered to be acceptable in statistical terms. Figure 1 shows the typical Bode spectra obtained and their respective adjustments.
To represent the measured electrode processes in the electrochemical double layer between the scaffolds, the physiological medium and the cells have assumed an equivalent circuit model that can present two variations depending on the adjustment to the spectrum obtained in each case and depending on the level of cell adhesion to the surface of the scaffold.
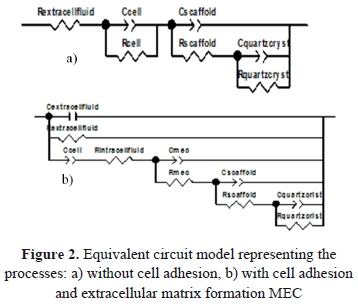
Figure 2. shows two equivalent electrical circuit models which have been proposed to adjust the impedance spectra obtained. Each model represents the electrochemical behavior associated with the interfaces: physiological fluid polymeric scaffold-cell-electrode. These interfaces are represented by the relaxation constants of charge transfer, related by a capacitance and resistance. The relaxations of transfer of the two electrode processes described above are related by a parallel circuit, due to the similarity in constant conductive (ion exchange), shown in the superposition of these in the respective spectra. This configuration is related to a series resistor representing the ohmic drop or opposition to the charge transfer from the electrolyte or cellular environment.
The first model (a) represents a situation where the cells do not develop on the surface of the scaffold, in which a relaxation time associated with cells in series with two parallel relaxations related to the interaction between the scaffold, the physiological medium, and the quartz crystal is presented.
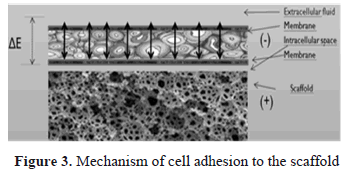
The second model (b) corresponds to the situation in which the cells have an adhesion to the substrate of the scaffold in addition to the formation of an extracellular matrix (ECM) produced by ion exchange from the scaffold to the physiological medium through the cells. Figure 3 shows a graphic diagram showing the mechanism of cell adhesion to the scaffold.
The second model represents a superposition of relaxation constants, which shows the behavior of the system: extracellular matrix-scaffold-cell-physiological fluid under ideal conditions for cell attachment. This model allows for one to represent the different variations of the resistive elements related to the effectiveness of cell adhesion on the surface of the scaffold under a polarization gradient. The correct setting in the simulation of the proposed model accurately validates the electrochemical behavior of the polarized system described.
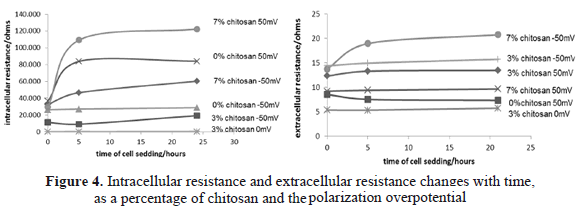
The extracellular fluid in contact with the cells and scaffold are represented by a capacitor and a resistor in parallel. An increase in this resistance is equivalent to a satisfactory cellular evolution. The capacitance of the cell corresponds to the evolution of endo-plasma membrane. The resistance of intracellular fluid is the transformation processes, adhesion and dendritic-cell breakdown, as well as an increase in ion transport across the cell membrane. A lower value of the resistance indicates a positive evolution of cell adhesion on the scaffold. Figure 4 shows the effect of the percentage of chitosan and polarization overpotential with respect to changes in extracellular resistance and intracellular resistance over time.
Electric fields have been detected in the healing of wounds and damaged tissues. The role of an electric field on the repair or regeneration of tissues remains an intriguing subject of research even today. Presumably, an applied electric field equivalent to an electric field magnitude measured in a process of cell regeneration in vivo or recovery of tissue activates many intracellular signaling pathways, possibly in a direction or patterns, as in the case of osteoblastic cells [16,17].
Measurement of intracellular resistance is a property of the cell indicating that the cell interface is split and that there is a broken endoplasmic membrane allowing the passage of extracellular fluid into the interior, increasing the transfer of electric charge by the passage of fluid minerals into cells [18].
Under these concepts, it was found that the variable electric overpotential had a greater influence on the intracellular resistance value compared to the variable concentration of chitosan. Negative overpotential values had a lower intracellular resistance which may indicate that they have a higher proliferation and cell adhesion. However, it is considered that very low values of intracellular resistance (results of the scaffold with 3% chitosan without polarization) may indicate ischemia or cell death.
The synergistic effect between the polarization potential and the percentage of chitosan may be better analyzed with the results of extracellular fluid resistance.
Figure 4 shows that more negative overpotentials are the highest values of resistance. Values less than 10 ohms could be considered indicators of cellular ischemia. As the concentration of chitosan, it is observed that at a lower concentration of chitosan, the cells showed a higher dielectric strength, which means less cellular evolution in adhesion and cell division [19].
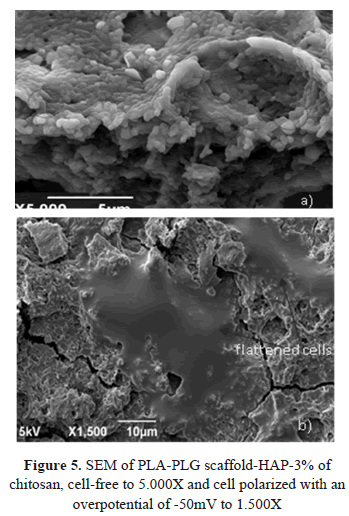
Figure 5 shows the SEM images of PLA-PLG scaffold-HAP-3% chitosan, cell-free to 5.000X and cell polarized with an overpotential of -50mV to 1.500X.
The scaffold of PLA-PLG-chitosan-HAP showed a structure with an interconnected porosity and irregular topography, characteristic of this type of materials used for cell regeneration, where these surface properties are essential to cell adhesion and proliferation. Figure 5a shows the scaffold without cells with the particular presence of hydroxyapatite (circles) on the surface, which improves cell adhesion.
Figure 5b shows the surface of the scaffold after a seed time of 24 h, with flattened and elongated cells with dendritic extensions, characteristic of proliferating cells. As hydroxyapatite and chitosan chemically similar to most organic and inorganic components of bone, they become excellent candidates to improve osteoconductivity and cell recognition in a composite scaffold. A scaffold of PLA-PLG-chitosan-HAP allows the growth of bone tissue as demonstrated in this work. The proposed scaffold biodegradation is reflected in a structural configuration of interconnected pores and irregular topography, favoring proliferation and adhesion, and allowing three-dimensional support cell growth [20].
4. CONCLUSION
A scaffold of polylactic acid, polyglycolic acid with additions of bioactive elements of chitosan (q), bioceramic (bc), and zinc has been subjected to cell-seeding experiments under the application of an overpotential. It is found that an applied cathode potential of this system produces a variation in the surface hydrophilicity and increases intracellular ion exchange. The cationic character acquired by the addition of zinc to the polymers and the cationic nature of the amino groups of chitosan in the scaffold were not the predominant effects on the increase in cell adhesion in these experiments. However, a greater amount of chitosan in the proposed scaffold promotes increased cell adhesion.
5. ACKNOWLEDGMENTS
This research was supported by COLCIENCIAS in the program of Technological Development and Industrial Innovation: Project Code 1102-403-20771.
REFERENCES
[1] Peter, X. M., Biomimetic materials for tissue engineering, Advanced Drug Delivery Reviews, 60, pp. 184-198, 2008. [ Links ]
[2] Joerg, K. T. and Achim, M. G., Matrices and scaffolds for protein delivery in tissue engineering, Advanced Drug Delivery Reviews, 59, pp. 274-291, 2007. [ Links ]
[3] Jeanie, L. D. and David, J. M., Hydrogels for tissue engineering: scaffold design variables and applications, Biomaterials, 24, pp. 4337-4351, 2003. [ Links ]
[4] Alexander, H., Nusret, S. G. and Aldo, R. B., A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics, Biomaterials, 32, pp. 2757-2774, 2011. [ Links ]
[5] Hanna, S., Samuel, I. S., Cellular response to zinc-containing organoapatite: An in vitro study of proliferation, alkaline phosphatase activity and biomineralization, Biomaterials, 26, pp. 5492-5499, 2005. [ Links ]
[6] Claire, CH. and Odile, D., Influence of the degree of acetylation on some biological properties of chitosan films, Biomaterials, 22, pp. 261-268, 2001. [ Links ]
[7] Hyeong-Ho, J. and Chang-Hun, L., In-situ formation of the hydroxyapatite/chitosan-alginate composite scaffolds, Materials Letters, 62, pp. 1630-1633, 2008. [ Links ]
[8] Joshi, R. P. and Sridhara, V., Microscopic calculations of local lipid membrane permittivities and diffusion coefficients for application to electroporation analyses, Biochemical and Biophysical Research Communications, 348, pp. 643-648, 2006. [ Links ]
[9] Shunichi, Y., Effect of intracellular Ca ion on the ion channel activities of squid axon membranes, Neuroscience Research Supplements, 9, pp. 75-83, 1989. [ Links ]
[10] Cortez, C. M. and Cruz, F. A., Influence of fixed electric charges on potential profile across the squid axon membrane, Physica B: Condensed Matter, 4, pp. 644-652, 2008. [ Links ]
[11] Morf, W. E. and Pretsch, E., Theoretical treatment and numerical simulation of potential and concentration profiles in extremely thin non-electroneutral membranes used for ion-selective electrodes, Journal of Electroanalytical Chemistry, 641, pp. 45-56, 2010. [ Links ]
[12] Hiroyuki, O., The Donnan potential-surface potential relationship for a cylindrical soft particle in an electrolyte solution, Journal of Colloid and Interface Science, 323, pp. 313-316, 2008. [ Links ]
[13] Groeneveld, E. H. and Burger, E.H., Bone Morphogenetic Proteins in Human Bone Regeneration, European Journal of Endocrinology, 142, pp. 9-21, 2000. [ Links ]
[14] Ahimou, V. M., Paquot, P., Jacques, P. T. and Paul, G. R., Influence of Electrical Properties on the Evaluation of the Surface Hydrophobicity of Bacillus Subtilis, Journal of Microbiological Methods, 45, pp. 119-126, 2001. [ Links ]
[15] Kristin, K. and Thomas, G., Surface modification of biomaterials to control adhesion of cells, Clinical Hemorheology and Microcirculation, 39, pp. 247-251, 2001. [ Links ]
[16] In, S. K. and Jong, K. S., Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts, Biochimica et Biophysica Acta, 1763, pp. 907-916, 2006. [ Links ]
[17] Cortez, C. M., Cruz, F. A. and Silva, D., Influence of fixed electric charges on potential profile across the squid axon membrane, Physica B: Condensed Matter, 403, pp. 644-652, 2008. [ Links ]
[18] Wang, E., Zhao, M., Regulation of tissue repair and regeneration by electric fields; Chinese Journal of Traumatology, 13, pp. 55-61, 2010. [ Links ]
[19] Pliquett, U., Joshi, R. P. and Sridhara, V., High electrical field effects on cell membranes, Bioelectrochemestry, 70, pp. 275-282, 2007. [ Links ]
[20] Carlos, G., Claudia, G. and Carlos, P., Formación in-situ de circonia en la síntesis de sustitutos óseos basados en matriz de alúmina-circón infiltrada con hidroxiapatita, Dyna, 77, 62, pp. 143-149, 2010. [ Links ]