Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.80 no.178 Medellín mar./abr. 2013
NITROGEN REMOVAL FROM LANDFILL LEACHATE USING A SEQUENTIAL BIOLOGICAL PASSIVE SYSTEM
REMOCIÓN DE NITROGENO DE LIXIVIADOS DE UN RELLENO SANITARIO MEDIANTE UN SISTEMA PASIVO BIOLOGICO SECUENCIAL
EDGAR SUÁREZ GARCÍA
Passant-Ph.D.Universidad Nacional de Colombia Medellín-Colombia, esuarez@unal.edu.co
SANTIAGO ALONSO CARDONA-GALLO
PosPh.D. Profesor Universidad Nacional de Colombia, Medellín- Colombia, scardona@unal.edu.co
Received for review June 1th, 2012, accepted November 6th, 2012, final version November, 30th, 2012
ABSTRACT: The efficiency of an anaerobic-aerobic biofilm passive system for nitrogen conversion was investigated. Leachate from real landfill is characterized by high ammonia content. Several techniques have been tested for ammonia nitrogen removal including air stripping, which imply high cost operation. In this work, an innovative design which combines a Bio- Reactive Permeable Barrier (BioBarrier) and multilayer Bio-trickling Reactor (BtR) was evaluated for nitrogen removal. The results show an excellent performance of BtR system, in which concentration of NO3 raise from 100±10 mg/L NO3 to near 1000±100 mg/L NO3. Almost 95% NH4 removal was observed, demonstrating efficiency of this device. However, N dynamics in BRPB had no important changes, probably due to low biofilm content of the package material. The BtR system was designed to promote O2 transference from the atmosphere without external energy input. Results confirm feasibility of nitrification process within tower. From the experimental data it can be concluded that BtR system is an efficient and economical system for ammonia removal, making it an innovative and potential system for small communities.
KEYWORDS: Nitrification, landfill leachate, passive system.
RESUMEN: Se estudio la eficiencia de un sistema pasivo de biopelícula anaerobio-aerobio para la remoción de nitrógeno. Se caracterizaron los lixiviados de un relleno sanitario con elevadas concentraciones de nitrógeno amoniacal, Diversas tecnologías se han aplicado para la remoción de nitrógeno, puede lograrse en sistemas con arrastre con aire, lo cual incrementa los costos elevados de operación. En este trabajo se evaluó la capacidad de remoción de nitrógeno con la implementación de un diseño innovador que combina una Bio-Barrera Reactiva Permeable (BioBarrera) y un Biofiltro multicapas (Bf). Los resultados presentan un adecuado desempeño del reactor Bf, en el cual la concentración de NO3 incrementó desde 100±10 mg/L hasta 1000±100 mg/L aproximadamente con una remoción del 95% para la especie NH4. Sin embargo la dinámica de las especies de N en el sistema BioBarrera no mostró cambios significativos, probablemente debido al bajo nivel de superficie del material de soporte disponible para la biomasa. La unidad Bf fue diseñada a partir de balances de masa para promover el intercambio gaseoso con el entorno sin requerir fuentes externas de energía. Los resultados experimentales confirmaron que el sistema Bf es una alternativa económica y eficiente para la remoción de amonio, haciéndolo ideal para pequeñas comunidades.
PALABRAS CLAVE: Nitrificación, lixiviado, sistema pasivo.
1. INTRODUCTION
Landfill leachates is a kind of highly contaminated wastewater made up after contact of rainfall and solid wastes. This fluid is rich in several contaminants including dissolved organic matter (volatile fatty acids, humic and fulvic acids), inorganic components (Ca2+, Mg2+, NH4+,NO3-, Cl-, HCO3-, SO3-), heavy metals (Cd, Cr, Cu, Ni, Pb, Zn) and xenobiotic organic compounds (halogenated hydrocarbons, aromatic hydrocarbons, phenols and chlorinated compounds). Volume and composition of leachates depends on site biogeochemistry, kind of waste disposed and age of landfill [1].
Treatability of landfill leachates depends upon composition and nature of organic matter, nutrients and other elements present. Most of available wastewater treatment methods have been applied, both biological and physicochemical. The most successful treatment usually requires several steps and treatment trains [2, 3].
Biodegradation of proteins and aminoacids inside landfills, leads to a production of high concentration of ammonia (or its ionic species ammonium under acidic conditions), specially for stabilized landfills. Ammonia toxicity impacts bacteria, algae, zooplankton and fish. Photosynthetic carbon fixation of marine diatoms was inhibited to almost 90% at an ammonia concentration of 55 mg/L. Ammonia has also shown to be toxic in oxidation ponds where high free ammonia (>36 mg L -1) and pH (>8.0) inhibit photosynthesis [4]. On the other hand, nitrates stimulate the growth of algae, contributing to the eutrophication of open bodies of water. Nitrites and nitrates may reach groundwater resources which are used for producing drinking water. High concentrations of nitrates and nitrites in drinking water cause methemoglobinemia in babies and promote the formation of carcinogenic nitrosamines [15]. Nitrogen compounds must therefore be removed from waste water.
Ammonium and nitrates removal from landfill leachates has been tested, namely air stripping [4], biological processes [2], precipitation and adsorption [7]. Removal of both ammonium and nitrates can be achieved efficiently through a sequence process, either by suspended or attached biological systems [5, 6]. The Permeable Reactive Barrier (PRB) is an effective alternative to traditional remediation methods for groundwater treatment. It has gained popularity because of its efficient removal of pollutants and low operating and maintenance costs (due mainly to its passive nature). However, most of the publications report the use of Zero-Valent Iron (ZVI), activated carbon, zeolites and limestone as filling material [8, 9, and 10].
The aim of this work was to investigate the performance of a sequential passive biofilm reactor, which includes a Permeable Bio-Reactive Barrier (BioBarrier) working under anaerobic conditions and a Multilayer Biotrickling Reactor (BtR) operated aerobically, for nitrogen related compounds removal from a real landfill leachate sample.
2. MATERIALS AND Y METHODS
2.1. Leachate CollectionLeachate from a local landfill site, receiving mainly domestic waste, was collected directly and transferred to the laboratory and maintained under refrigerated conditions (4°C) in plastics containers, prior to subsequent tests. Its major characteristics of importance for this work are shown in table 1.
2.2. Experimental devices
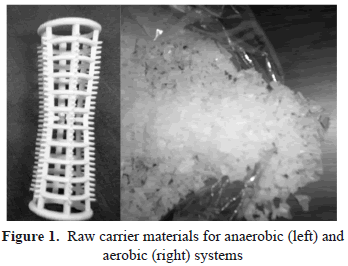
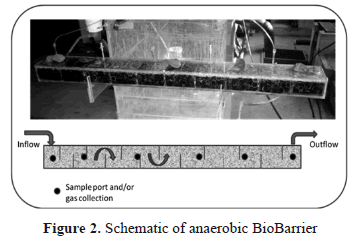
Experimental studies were conducted in order to investigate N-species performance under both anaerobic and aerobic conditions in a passive-biofilm reaction system. The plastic carrier materials used for the reactor are presented in figure 1. Sequential treatment starts from the Anaerobic BioBarrier (figure 2) and finishes in the Aerobic multilayer Biotrickling Reactor (BtR figure 3).
The anaerobic reactor was designed as a PRB, consisting of a horizontal-rectangular acrylic column with a sectional area of 50cm2 and 1m length, and almost 95% working volume. Startup time was selected to be 1 month in accord with previous investigations. Anaerobic sludge, taken from a local Wastewater Treatment local Plant (WWTP), was fed into a tank containing carrier material and diluted raw leachate was pumped out.
Aerobic BtR was designed to provide enough dissolved oxygen for nitrification process. As an alternative aeration mechanism, a multilayer packed bed framework was built from mass balances; between each bio-reactive zone, an aeration zone allows oxygen to diffuse inside water. For its design, a limit for nitrification was selected as 1mg DO/L (Dissolved Oxygen/L) with dispersion of drops across rectangular sectional area avoids the saturation of the bed and increases mass transfer efficiency. Effluent from the BioBarrier was injected directly into the BtR. Other units make up the complete treatment train system (not investigated). Influent for the BioBarrier comes from a settler unit and effluent from the BtR goes to a wetland unit. Aerobic sludge was fed into each BioReactive zone, being supplied as diluted raw leachate.
Leachate was kept in a regulation tank before treatment. The first step included a primary settler and then it was transported through a pipe to the BioBarrier. A valve was used to maintain flux at a constant rate of 1L/day. The temperature did not show significant change over the 60 day experiment.
2.3. Analytical methods
NH4+-N and NO3-N species were measured using an Ammonium electrode and a Nitrate electrode, model 250 Denver Instruments. pH was measured using a Denver Instrument pH-meter. Dissolved oxygen was determinate using an oxygen-meter OD-HQ 400 (LO 101-01 electrode).
3. RESULTS AND DISCUSSION
An attached growth system is generally considered less sensitive to toxicity and variations in environmental conditions, additionally, provide correct mixing of the reactor contents resulting in an efficient mass transfer [3]. Three major biological processes are directly involved with biological nitrogen removal in wastewater treatment: assimilation, ammonification, nitrification/denitrification and anammox method [3].
3.1. DO and pH behavior in BioBarrier-BtR system.
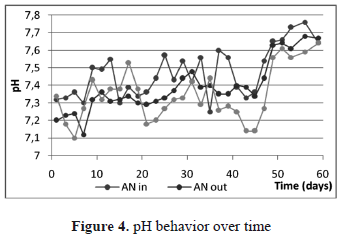
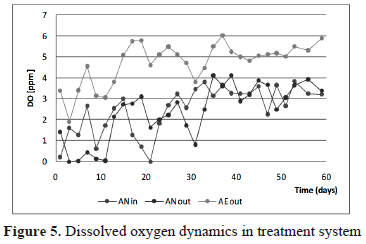
In figures 4 and 5 pH and DO behavior is shown. According to table 1, the leachate sample should correspond to an "old leachate" which means, the biodegradation inside the landfill currently belongs to a methagenic phase, and production of organic acids is low and/or alkalinity production could avoid a pH drop. Almost neutral conditions were observed during the experiment, indicating also good self-buffering capacity in both reactors, and feasibility for most biological processes.
Where AN in: inflow anaerobic process. AN out: outflow anaerobic process.
Where AE out: outflow aerobic process
The DO at the inflow of the BioBarrier, there the leachate exhibited an aerobic condition all the time. As was mentioned above, before the BioBarrier, a settler unit was used in order to reduce particulate material entering the system. That unit, built of transparent acrylic, could engender metabolic and photosynthetic activity which would lead to a high increase in oxygen content.
In the BtR unit, however, experimental evidence confirms previous mass balance design. The natural and passive supply of oxygen could be achieved efficiently during operation, increasing DO concentration roughly from 2-3 to 5-6, allowing any nitrification activity.
3.2. Ammonium dynamics
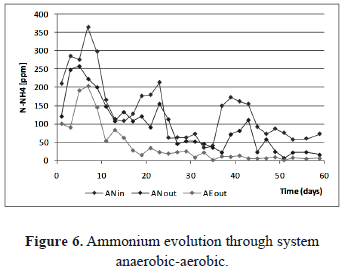
The removal of ammonium in the system during operation is shown in figure 6. Initial leachate conditions could be maintained, the effect of settler also affected the N-NH4+ concentration before BioBarrier, indicating some removal capacity. Ammonia removal was possible even with no air injection. In fact, [4] reported near to 70 percent of total loss due to desorption through the water surface; air stripping improved removal to near to 90%.
Ammonium or ammonia levels depend on pH. At pH values higher than 9, the ammonia species dominate, while at a pH of 7 or below, ammonium is present in bigger proportion. At pH values of 8, approximately the same amount of both species is present. According to figure 4, during operation NH4+ dominates against toxic NH3 [16]. In an anaerobic process, ammonium reacts with carbonates to form ammonium bicarbonate, which helps to maintain the pH values stable. If some acidogenic and acetogenic activity is present, this system prevents pH fall [17]. When ammonium is used for growth by microbial strains, it is absorbed easily and incorporated into organic nitrogen through alanine metabolism and then used for building the cellular structure [17].
In figure 6, the ammonium behavior in leachate entering the anaerobic reactor indicates both a physical and a biological process in the settler unit. Peak values in the first week indicate possible lyses processes which led to an increase in NH4+ specie [16]. Ammonia transformation includes assimilation, oxidation towards nitrite and nitrate and oxidation to N2 [16].
In the BioBarrier unit, NH4+ was slightly removed during almost all the operation. The probable mechanism under anaerobic conditions is assimilation, but as a natural and passive system (with no energy and chemical input), aerobic conditions dominated always. From 3-7 days, depletion of oxygen inside the BioBarrier indicates bacterial activity, but not ammonium oxidation (figure 7). Experimental evidence confirms the operation of both systems under aerobic condition.
However a novel promising low cost alternative to ammonium removal could be responsible for NH4+ removal within a few days under anaerobic conditions in the first stages. The Annamox process or anaerobic oxidation of ammonium uses nitrite as an electron acceptor and ammonium as an electron donor, producing nitrogen gas. Even though Annamox process could explain the consumption of NH4+, the long start up times, required for that process, do not support this hypothesis [3].
After the first ten days, removal of ammonium occurred under aerobic conditions. The main mechanism must correspond to nitrification, but according to figure 7, nitrate in the BioBarrier reactor remains almost constant over time. This could be attributed to a carrier material, which provides low surface area for the nitrifying microbial biofilm development.
Physical desorption and accumulation in gas collection bags should explain ammonium removal inside the BioBarrier [4.12]. The concentration of ammonium was refined in the BtR unit mainly by nitrification. According to figures 4 and 5, both the pH and the DO conditions favored oxidation of NH4+ to NO3+. Note how the concentration of ammonium was kept under permissible levels after day 35, with almost complete removal efficiency (figure 6).
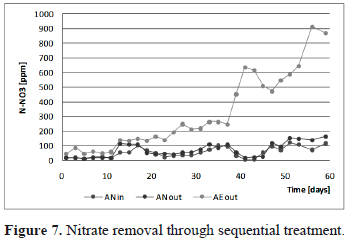
3.3. Nitrate dynamics
Evolution of NO3- is shown in figure 7. The processes of nitrification (the oxidation of ammonia to nitrate by the Nitrosomonas and the Nitrobacter species microbial strains) and denitrification (the reduction of nitrate to nitrogen gas) are two major reaction pathways in the natural nitrogen cycle [12]. Nitrification is the autotrophic, sequential oxidation of the ammonium ion (NH4+), first to nitrite (NO2-) and then to nitrate (NO3-), while denitrification is the heterotrophic, anoxic two-step conversion of nitrate, first to nitrite and then to gaseous nitrogen compounds [11].
Nitrate concentration before the BioBarrier system remains constant for the first eleven days. Then, nitrate shows ups and downs reaching a maximum value near 180 [ppm] at the end of experimentation. This behavior corresponds, probably with the ammonium decrease presented in figure 6, and supports the idea of aerobic activity before the BioBarrier unit.
The autotrophic bacterium oxidizes inorganic nitrogen components to obtain energy for growth and maintenance, while they obtain carbon for cell building by the reduction of CO2 [16]. The nitrification rate is limited entirely if oxygen is not supplied. The optimum pH for the growth of nitrifying bacteria is generally assumed to be pH 7.2-8.0 and DO must be maintained above 2.0 [ppm] (or 1 [ppm] for nitrite conversion) [3]. From figures 4 and 5, ideal conditions were self-maintained during most of the experimental time above after day 35. During days 3 to 11 and 29-31, it is expected that first aeration zone provides OD for nitrification. A low wastewater pH has the primary effect of inhibiting nitrifiers' enzymatic activity and has a secondary effect on the availability of alkalinity. NH4+ inhibits nitrification above 400-500mg/L [16, 3]. According to experimental data, both the pH and ammonium concentration remain at levels that allow efficient nitrification within the BtR unit. This also indicates that enough supply of inorganic carbon as bicarbonate (HCO3-) was maintained in the final step and not in the BioBarrier system in which aerobic conditions were also favorable for nitrification [12].
Aerobic conditions and the pH in the BioBarrier reactor suggest that an increase in NO3- concentration should be observed, but this species remains almost constant over time. This indicates that probable incomplete ammonium oxidation occurred, presumably as a result of low concentration of carbonate alkalinity (∼2 [ppm]), which may cause a limitation in the ammonium oxidation rate of nitrifiers [14]. This is because when treating high ammonia landfill leachates characterized by low biodegradable organic levels, a supplementary source of organic carbon is required to ensure adequate denitrification. As it was mentioned, when no other external source of nutrients was added denitrification did not take in the system [11].
Another factor that could increase efficiency of nitrification was a carrier media. The biocarrier used for BtR exhibits better characteristics such as higher specific area, better roughness and low size. The surface characteristics of the carrier are of great importance for the performance of treatment plants. This is most likely explained by the rougher surface of the carrier resulting in an enlargement of the biofilm surface on a "micro scale" [13]. The efficiency of nitrification was noticeable. A maximum nitrate increase was observed from concentrations reaching 150 [ppm] to concentrations near 900 [ppm], with a consequent reduction of ammonium concentration below 10 [ppm] (figures 6 and 7).
4. CONCLUSIONS
Anaerobic conditions inside the first reactor could not be maintained due to the previous settler step in which possible photosynthetic activity increased dissolved oxygen prior to the BioBarrier. Nitrate concentration remains almost constant throughout the BioBarrier, due probably to low biological activity and the lack of carbonate alkalinity. Both systems were efficient in ammonium removal, and conditions of pH and dissolved oxygen were self-maintained. The BtR unit was highly efficient in the nitrification of ammonium, showing that passive aeration zones provides enough oxygen for the nitrification process and has Bioreactive zones with ideal conditions for ammonium oxidation. The results show an excellent performance of BtR system, in where concentration of NO3 raise from 100±10 mg/L NO3 to close 1000±100 mg/L NO3. Almost 95% NH4 removal was observed, demonstrating efficiency of this device. However, N dynamics in BRPB had no important changes, probably due to low biofilm content of the package material. The BtR system was designed to promote O2 transference from the atmosphere without external energy input. Results confirm feasibility of nitrification process within tower. From the experimental data it can be concluded that BtR system is an efficient and economical system for ammonia removal, making it an innovative and potential system for small communities.
5. RECOMENDATIONS
The study developed allows recommending for future research the change sequence of reactors, as anaerobic, anoxic, aerobic and facultative. Research allows suggestion avoid initial settler for evaluation of anaerobic reactor and potential remove to nitrogen. Finally, is possible include final step wetland for NO3- high concentration removal.
ACKNOWLEDGMENTS
COLCIENCIAS con Grupo Red de Cooperación en Investigación Sobre el Agua-GRECIA Project. Facultad de Minas, Faculta de Ciencias, Universidad Nacional de Colombia Sede Medellín.
REFERENCES
[1] Giraldo, E., Tratamiento de lixiviados de rellenos sanitarios: avances recientes. Revista Facultad de Ingeniería Universidad de los Andes, Vol. 14, pp. 44-55, 2001. [ Links ]
[2] Wiszniowski, J., Robert, D., Surmacz, J., Milksh, K., Weber, J., Landfill leachate treatment methods: A review. Environ Chem. Lett, Vol. 4, pp. 51-61, 2006. [ Links ]
[3] Wiszniowski, J., Robert, D., Gorska, J., Miksch, K., Weber, J.V., Landfill leachate treatment methods: A review. Environ Chem. Lett. Vol. 4, pp. 51-61, 2006 [ Links ]
[4] Cheung, K. C., Chu L.M., Wong, M. H., Ammonia stripping as a pretreatment for landfill leachate. Water, Air, and Soil Pollution, Vol. 94, pp. 209-221, 1997. [ Links ]
[5] Welander, L.U., Henryssow, T., Welander, T., Nitrification of landfill leachate using suspended-carrier biofilm technology. War. Res., Vol 31, (9), pp. 2351-2355, 1997. [ Links ]
[6] Sheng, C., Dezhi, S., Shik, C.J., Simultaneous removal of COD and ammonium from landfill leachate using an anaerobic-aerobic moving-bed biofilm reactor system. In press. Waste Management. 2007. [ Links ]
[7] Karadag, D., Tok, S., Akgul, E., Turan, M., Ozturk, M., Demir, A., Ammonium removal from sanitary landfill leachate using natural G¨ordes clinoptilolite. Journal of Hazardous Materials. Vol. 153, pp. 60-66, 2008. [ Links ]
[8] Gavaskar, A.R., Design and construction techniques for permeable reactive barriers. Journal of Hazardous Materials. Vol. 68, pp. 41-71, 1999. [ Links ]
[9] Blowes, D.W., Ptacek, C.J., Benner, S.G., McRAE, C.W.T., Bennett, T.A. and Puls, R.W., Treatment of inorganic contaminants using permeable reactive barriers. Journal of Contaminant Hydrology. Vol. 45, pp. 123-137, 2000. [ Links ]
[10] Hunter, W.J., Shaner, D.L., Nitrogen limited biobarriers remove atrazine from contaminated water: Laboratory studies. Journal of Contaminant Hydrology. Vol. 103, pp. 29-37. 2009. [ Links ]
[11] Ilies, P., Mavinic, S., Biological nitrification and denitrification of a simulated high ammonia landfill leachate using 4-stage Bardenpho systems: system startup and acclimation. Can. J. Civ. Eng. Vol. 28, pp. 85-97, 2001. (11) [ Links ]
[12] Parkes, S., Jolley, D., Wilson, S., Inorganic nitrogen transformations in the treatment of landfill leachate with a high ammonium load: A case study. Environ Monit Assess. Vol. 12, No. 4, pp. 51-61, 2007. [ Links ]
[13] Welander, U., Henryssow T. and Welander T., Nitrification of landfill leachate using suspended-carrier biofilm technology. War. Res. Vol. 31, (9), pp. 2351-2355, 1997. [ Links ]
[14] Chen, S., Sun, D. and Chung, J.S., Simultaneous removal of COD and ammonium from landfill leachate using an anaerobic-aerobic moving-bed biofilm reactor system. Waste Management. In press. 2007. [ Links ]
[15] Zhengyong, X., Zhaohui, Y., Guangming, Z., Yong, X., Jiuhua, D., Mechanism studies on nitrogen removal when treating ammonium-rich leachate by sequencing batch biofilm reactor. Front. Environ. Sci. Engin. China. Vol. 1, (1), 43-48, 2007. [ Links ]
[16] Wiesmann, U., Choi, I.S., Dombrowski, E.M., Fundamentals of Biological Wastewater Treatment. Wiley VCH. 2007. [ Links ]
[17] Bitton, G., Wastewater microbiology. Third edition. John Wiley & Sons, 2005. [ Links ]