Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.80 no.178 Medellín mar./abr. 2013
MELT-PROCESSING OF SBS/POLYANILINE-BASED BLENDS
PROCESAMIENTO TÉRMICO DE MEZCLAS A BASE DE SBS Y POLIANILINA
RICARDO HERBÉ CRUZ-ESTRADA
PhD. Ing. Químico Industrial, Centro de Investigación Científica de Yucatán, México Unidad de Materiales, rhcruze@cicy.mx
CARLOS ROLANDO RÍOS-SOBERANIS
PhD. Químico Industrial, Centro de Investigación Científica de Yucatán, México Unidad de Materiales, rolando@cicy.mx
CARLOS VIDAL CUPUL-MANZANO
MSc. Ing. Químico Industrial, Centro de Investigación Científica de Yucatán, México Unidad de Materiales, ccupul@cicy.mx
Received for review February 24th, 2012, accepted September 11th, 2012, final version November, 15th, 2012
ABSTRACT: Morphological analysis and electrical conductivity of composites obtained by melt-processing of blends of a polyaniline complex (PANICX) with styrene-butadiene-styrene (SBS) are reported. Blends with PANICX contents ranging from 1 to 50 wt% were first prepared using a two-piece batch mixer, followed by ram-extrusion to obtain cord-like extrudates. The microstructure was analyzed by SEM, and conductivity was evaluated with a Keithley electrometer. The blends' rheological behavior was also studied. Evidence of formation of well-defined elongated structures was found in most of the extrudates. The extrudates' conductivity was considerably low. Inhomogeneity-related problems were observed such as erratic behavior of pressure, "melt-fracture" during the extrusion, and extrudates with irregular microstructure and very low conductivity. This occurred because same size batches were used regardless of blends' composition. As a result, the shearing occurring during compounding was not enough to disperse PANICX effectively within the SBS matrix.
KEYWORDS: Conducting polymers, Polymer blends, Extrusion, Microstructure, Polymer processing.
RESUMEN: Se reportan microestructura y electro-conductividad de materiales obtenidos mediante procesamiento térmico de mezclas de un complejo de polianilina (PANICX) y SBS. Mezclas con 1-50 % en peso de PANICX se prepararon primero en cámara de mezclado, seguido de extrusión ram. El comportamiento reológico se estudió durante la extrusión, la microestructura fue analizada (SEM) y se evaluó la conductividad (electrómetro Keithley). Se encontró evidencia de formación de estructuras alargadas bien definidas en la mayoría de los extrudidos. La conductividad fue considerablemente baja. Problemas relacionados con falta de homogeneidad se observaron: comportamiento errático de presión, fractura de fundido, y obtención de extrudidos con microestructura irregular y muy baja conductividad. Esto ocurrió debido a que lotes del mismo tamaño fueron usados independientemente de la composición de las mezclas; lo que propició que los niveles de esfuerzos cortantes generados durante la extrusión no fueran suficientes para dispersar eficazmente PANICX en el interior del SBS.
PALABRAS CLAVE: Polímeros conductores, mezcla de polímeros, Extrusión, Microestructura, procesamiento de polímeros.
1. INTRODUCTION
Intrinsically conducting polymers (ICPs) are an important class of polymeric materials because they present some properties that are not found in conventional polymers; for instance they have excellent electrochemical properties, and can be produced by following many different routes, one of them being electrochemical oxidation [1]. In spite of this, ICPs are known to have poor mechanical properties that render them as non-ideal materials to be used on their own for practical applications. Another important issue is related to their processability. In this respect, over the years the general knowledge has been that they cannot be easily melt-processed. Nowadays many of the problems above mentioned have been solved. The lack of good mechanical properties has been overcome by blending the ICPs with traditional polymers with superior mechanical properties. On the other hand, the processability problems have been considerably reduced by using ICPs-based complexes containing processing aids to reduce the viscosity levels of blends during the melt-processing. Blends of insulating polymers with ICPs can also be obtained by "in situ" chemical or electrochemical polymerization in the presence of an insulating polymer, or by blending a soluble ICP with another polymer in solution [2,3]. A wide-open horizon of potential applications has emerged for conducting polyblends. For instance great advances have occurred in the storing of energy [4], the protection from electromagnetic interference [5], the design of electro-optical devices [6], the welding of plastics [7], the fabrication of transparent conducting films [8], antistatic textiles [9], and membranes for electrodialysis [10], just to mention a few. One of the main problems that scientists and technologists face during the melt-processing of polymer blends is the difficulty to obtain a homogeneous final product. In this respect, among many other aspects that need to be considered, the "fill factor" plays an important role because it helps to select the adequate batch size in order to produce a homogeneous blend [11]. As part of a broad program of work aimed at studying the production of aligned ICPs structures, formed during the blending with different insulating polymer matrices by melt-processing, the morphology and conductivity of the resultant composites have also been examined, which could be used in most electronic applications against electrostatic discharge. For this work, an experiment with a polyaniline (PANI) complex and a viscous triblock copolymer of styrene-butadiene-styrene (SBS) was performed. The SBS functions advantageously when it is used as the insulating matrix due to its elastomer-thermoplastic nature, i.e. it presents good mechanical properties as an elastomer material without needing any additional process and can be processed as a thermoplastic. Additionally, it is believed that its high viscosity could favor deformation of the PANI complex phase into elongated structures due to an effective stress transfer, thereby aiding the drawing process. With respect to PANI, it may have the greatest potential for commercial applications, being one of the most widely used ICPs in the preparation of blends with conventional polymers due to its unique electrochemical properties, good environmental stability, easy polymerization, and low costs [12-14]. This paper reports on the experience obtained when dealing with the melt-processing of SBS/PANI-based blends. It also considers the morphology and the conductivity of the resultant composites.
2. EXPERIMENTAL
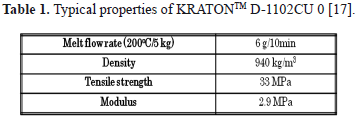
A PANI complex (PANICX) provided by Panipol Ltd. was used as filler material (grade CX100X03). It has an approximate composition of 25 weight % (wt%) of electro-conducting PANI and 75 wt% of zinc dodecyl-benzene sulphonate [15,16]. This zinc compound has surfactant properties that lower the viscosity of the PANICX polyblends when they are melt-processed. It was used as received, in the form of pellets, and its typical density and volume conductivity are 1100 kg m-3 and 10-4 S cm-4, respectively. A Polystyrene-Polybutadiene-Polystyrene block copolymer (SBS) was used as the matrix material. It was provided by Shell Chemicals and is identified as KRATON™ D-1102CU (formerly Cariflex TR-1102). The polymer is a linear three-block copolymer consisting of chains of the form polystyrene (PS)-polybutadiene (PB)-polystyrene (PS). Typical properties are presented in Table 1.
In previous publications [18] it has been reported that the viscosity level of the SBS is considerably higher than that of the PANICX. Their rheological behaviour was investigated by capillary rheometry to obtain shear viscosity vs. apparent wall shear rates relationships at 120oC following the Poiseuille Law for capillary flow.
2.1. Blends preparation
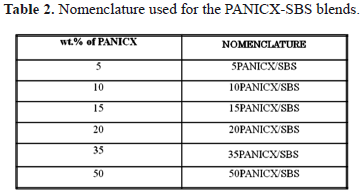
The materials were melt-blended at 130oC and 15 rpm for 5 min using a two-piece batch mixer (capacity = 60 cm3) attached to a torque rheometer instrument (BRABENDER® PLASTI-CORDER® PLE 330). Blends with 5, 10, 15, 20, 35 and 50 weight percent of the PANICX were prepared. Batches of 40 g were used in the preparation of all the blends for practical purposes. In addition, it was considered that this would ensure the operation of the instrument within its safety limits, that is, at torque levels no higher than 50 Nm. The nomenclature used throughout the text to identify the blends is indicated in Table 2.
2.2. Blends processing
Before processing the blends, they were chopped with an USI-Cumberland granulating machine (Model 5X7) that had a metal screen plate drilled with holes of ca. 3 mm in diameter. Each blend was then melt-processed by extrusion using the same instrument employed to investigate the rheological behaviour of the materials. That is, a Davenport ram-extrusion rheometer (piston diameter = 19.05 mm) and capillary die with a length (L) = 35 mm and a radius (r) = 0.8 mm. The blends were extruded at 120oC, using a piston drive speed of 1.25 mm/min that forced the melt at a constant rate through the capillary die. A transducer situated near to the entrance of the capillary die sensed the pressure of the melt so the behaviour of pressure with respect to time for each blend was registered.
2.3. Morphological analysis
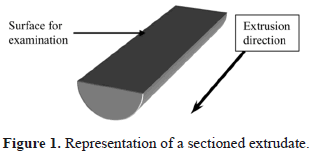
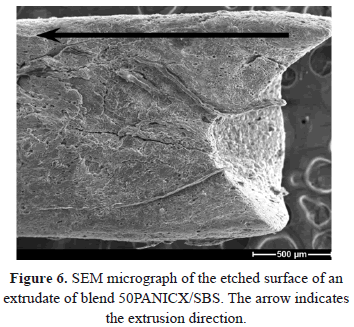
The morphology of the extruded PANICX-SBS blends was investigated by scanning electron microscopy (SEM) using a JEOL JXA-840-A electron probe microanalyzer at 10 kV. The specimens studied were the extrudates longitudinal sections etched with a mixture of chromic and phosphoric acids, which mostly removed the PANICX phase from the surface of the specimens and left the SBS phase exposed. Details of the etching procedure are reported elsewhere [15]. All the sections used for the analysis were obtained by longitudinally cutting the extrudates into halves in such a way that flat surfaces parallel to the extrusion direction, as close as possible to the extrudates' central axis, were obtained for etching and further examination (Fig. 1). Extrudates of blends 10PANICX/SBS, 20PANICX/SBS and 50PANICX/SBS were selected for the analysis. They were gold coated for 4 min in an E5000 (Polaron Equipment Limited) SEM coating unit.
2.4. Conductivity measurements
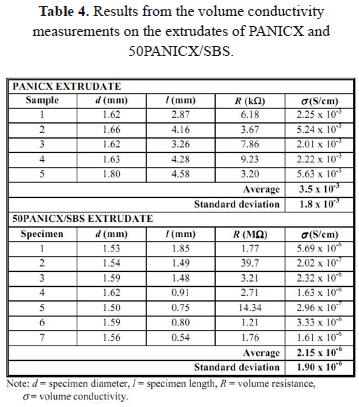
The volume conductivity (s) of the extruded PANICX/SBS blends along their main axial direction was evaluated by measuring the extrudates' electrical resistance using a Keithley 614 electrometer. For each extrudate, five to ten specimens, whose length ranged from about 0.5 to 2 mm, were tested using mercury as the electrical probes according to the method described by Cruz-Estrada et al. [15,16]. The specimens were cut at random from different sections of the extrudates. PANICX samples extruded under the same conditions to those used for melt-processing the blends were also evaluated. The extrudates' sections produced at the beginning of the extrusion process (stabilization stage) were excluded from the analysis. Assuming that the resistance readings were performed at an ohmic regime and that the specimens were perfect cylinders, the volume conductivity was calculated using the specimens' dimensions and (1) [19]:

where s is the specimen volume conductivity, l is the specimen length, A is the specimen cross sectional area, R is the specimen volume resistance, and d is the specimen diameter.
3. RESULTS
3.1. Blends processing
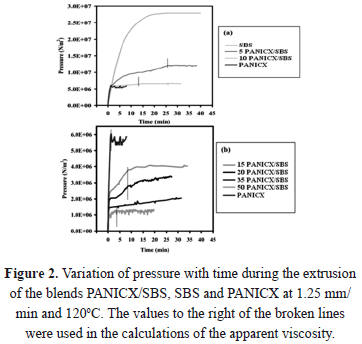
The pressure behavior with respect to time for each blend is presented in figure 2 (note the difference in the pressure-axes scale). The variation of pressure with time for the SBS and PANICX, processed at the same extrusion conditions to those used for the processing of the blends, is also depicted in figure 2 for comparison purposes. As can be noticed, during the extrusion of the blends the pressure did not display the characteristic behavior observed during the extrusion of a homogeneous material (e.g. SBS), that is, a gradual increase with time up to a level at which it remains constant. On the contrary, the pressure gradually increased up to a certain value and then started to display erratic behavior, which suggests that the blends were not completely homogeneous. In fact, this behavior appeared to increase, by increasing the PANICX content, which may suggest that the homogeneity degree of the blends decreased in the same manner. The latter trend may be due to the fact that batches of the same size were used during the blends preparation for compounding, regardless of the blends composition. To reduce the occurrence of inhomogeneity-related problems, the adequate batch size was estimated since it is actually a variable of the blend composition. As a matter of fact, (2) it is recommended to estimate the batch size [11]:
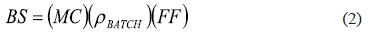
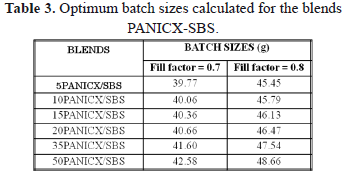
where BS is the batch size, MC is the mixer capacity, sbatch is the density of the final fluxed batch, and FF is the fill factor. By definition, FF is the fraction of the total volume of a mixing chamber that a final fluxed batch should occupy in order to produce a homogeneous blend, and typically ranges from 0.7 to 0.8 [11]. Now, equation (2) is useful to demonstrate that the batch size (i.e. 40 g) actually used in this work for all the blends during the compounding process was not strictly the correct one. Accordingly, Table 3 presents the optimum batch size corresponding to each blend. For the calculations, the maximum and minimum values recommended for FF were used in (2), that is, 0.7 and 0.8. To estimate rbatch, the density values for PANICX and SBS were used following the rule of mixtures.
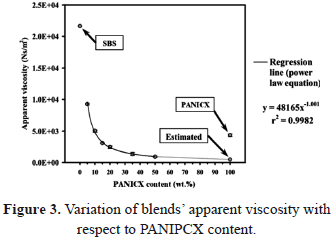
As can be seen in Table 3, the optimum batch size corresponding to each blend should have increased with the increase of PANICX in the blends. Additionally, it is evident that the difference between the batch size actually used and the optimum batch size also increased with the increase of PANICX content. This caused the level of shearing required to effectively disperse the conductive complex within the SBS phase in the melt to decrease in the same fashion, which in turn resulted in a reduction of the blends homogeneity. The experiment was not repeated using the optimum batch sizes because previous trials using more than 40 g caused torque levels higher than 50 Nm, which is the safety limit to operate the instrument without damaging it. The erratic behavior of pressure observed during the extrusion of the blends (Fig. 2) could be explained as follows: As the blends are not homogeneous and since the PANICX is considerably less viscous than the SBS, on emerging from the capillary, portions of the fluid melt rich in PANICX, would generate a sudden drop in the level of pressure sensed by the transducer. On the other hand, portions of the blend rich in SBS would generate a sudden increase in the pressure. As the melt was constantly flowing through the capillary, it generated the intermittent effect observed in the pressure-time curves. In spite of this, to the naked eye most of the extrudates had reasonable good appearance. That is, their surface finish was smooth and showed a glossy texture. Defects were observed, however, mostly in extrudates of blends 35PANICX/SBS and 50PANICX/SBS, as they presented sections that were brittle. This was observed especially in the extrudates of the latter blend, in which the brittle sections were more abundant. What is more, it was not even possible to obtain a continuous extrudate with no holes and breaks. To the naked eye it was easily observed that the extrudate was divided into short sections of about 3-5 mm long, which were connected by very weak "ring-like" features, which in turn caused the sections to break very easily during handling. That is why there was no need to use a magnifying or light-collecting optical device. It is believed that these ring-like "connectors" belong to portions of the flowing melt that were rich in PANICX, which generated the sudden drops in the level of pressure observed in figure 2b. On the other hand, the opposite should apply to the "well-connected sections", which corresponds to rich-SBS regions. The phenomenon observed is characteristic of melt fracture occurring in the flow of the melt during processing. Since at a fixed extrusion speed and temperature, melt fracture was most evident at higher concentrations of PANICX that negatively affected the blends homogeneity degree; this implies that melt fracture should be a function, not only of the extrusion speed, but also of the blends homogeneity. In other words, the less homogeneous the blends are, the more possibilities for melt fracture to occur. A corollary to the above observations is that in order to obtain extrudates of improved quality, the blends homogeneity degree must be as high as possible, and for this, the correct batch size must be used in their preparation. It is interesting to notice, however, that contrary to what it was expected, the level of pressure generated during the extrusion of the PANICX pellets was higher than that generated by the blends with 15, 20, 35 and 50 wt.% of conductive complex processed under the same conditions (Fig. 2b), which implies that the pellets are more viscous than some of their blends. This effect is better illustrated in fig. 3, which shows the variation of the apparent viscosity with respect to PANICX content. The data presented in the figure was calculated using the Poiseuille Law for capillary flow, the dimension of the capillary die and piston ram (19.05 mm in diameter), the piston drive speed (V = 1.25 mm/min) and the maximum level of pressure generated during the extrusion of each material. The equation to calculate the apparent viscosity (h) is the following:
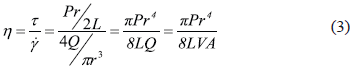
where g is the apparent wall shear rate, t is the wall shear stress, P is the pressure at the entrance of the capillary, r is the capillary radius, L is the capillary length, Q is the volume flow rate, V is the piston drive speed, and A is the area of the piston ram. The extrusion of blends PANICX/SBS, SBS and PANICX was performed at 1.25 mm/min and 120oC. For this, a capillary die with an L = 35 mm and an r = 0.8 mm was used. During extrusion, the pressure sensed by a transducer, situated near to the entrance of the capillary die, was recorded for the calculations. An average value was used when the pressure displayed erratic behavior, this was calculated for each case considering all the values on the right-hand side of the broken lines shown in Fig. 2. The apparent viscosity of the pellets of PANICX, together with that of the SBS are depicted in Fig. 3 as 100 and 0 wt.% of conductive complex, respectively (i.e. the blue marks).
As commented above, it would be expected that the PANICX pellets should be less viscous than their corresponding blends. The level of viscosity that one would expect would be around the value indicated in figure 3, that is, the red circle at 100 wt.% of PANICX content. This value was calculated using a power law equation (4), which described the behavior of the experimental data with a considerably high degree of accuracy (hence the high value of the squared correlation coefficient, r2, of 0.9982). It should be mentioned that (4) does not describe a rheological behavior, it was merely used to fit the experimental data in order to estimate the level of viscosity expected for the PANICX pellets.
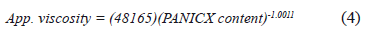
The situation depicted in Fig. 3 is due to the fact that the blends were subjected to two processing cycles (blending for compounding and forming, respectively), whereas the pellets of PANICX were subjected to only one (forming). Such an explanation is supported by the fact that the shearing history (among other factors) of the polymer melt affects its viscous flow during processing. As the flow occurs, the polymer macromolecular chains slide past each other. Therefore, the ease of flow will depend on the mobility of the molecular chains, and the forces or entanglements holding them together. Since both the SBS and PANICX constituting the blends were pre-sheared during the compounding process, it is very likely that some of the blends were less viscous than the pellets of PANICX due to a macromolecular chain disentanglement effect occurring in both the SBS and PANICX. Additionally, it should be considered that the blends were also subjected to a size reduction process in a granulating machine in which they were exposed to very high levels of impact and shear, which should have contributed to the occurrences illustrated in Fig. 3. It has been evinced that the morphology of the pellets of PANICX consists of globular-shaped agglomerates formed of primary dodecylbenzene sulphonic acid (DBSA)-doped PANI nanoparticles [15,16], which may be composed of highly entangled macromolecular chains. Therefore, it is possible that chain disentanglement occurred upon shearing during the processing stages. In consequence, it may also be possible that the DBSA-PANI macromolecular chains were oriented in the direction of the extrusion during the blends' melt-processing. In one way or another, different researchers have also studied the rheological behavior of other polyaniline-based systems. For instance, Joon-Sung et al. [20] give an account of the rheological characterization of the gelation process of solutions of polyaniline-emeraldine base/N-Methyl-2-pyrrolidone. In this particular case, the gelation process was characterized by measuring the viscoelastic property in an oscillatory test. The aforementioned investigators report that the solutions showed a weak gel state in which the storage modulus decreased rapidly even at low strain in the constant angular frequency test, and the damping factor increased rapidly to a state of typical viscous liquid at a specific angular frequency in the early stage of the constant strain test. They point out that the weak bonding of dipole force between polymer molecules was broken by the external deformation. They defined the angular frequency of the sudden change in the damping factor as a critical angular frequency. Moreover, the polyaniline-based solutions with a prior shear deformation resulted in the lower critical angular frequency, showing the delay of the gelation process by the prior shear deformation.
3.2. Morphological analysis
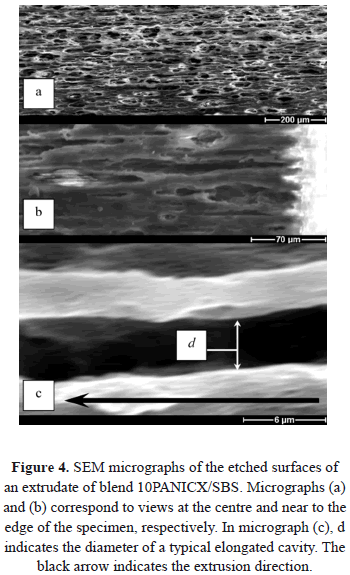
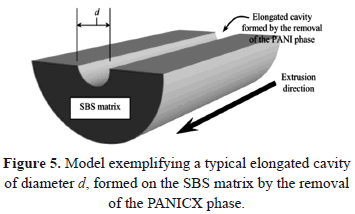
Most of the surfaces of the specimens examined displayed elongated cavities formed by the removal of PANICX, which was washed away (dissolved) by the acidic solution during the etching. The presence of elongated cavities increased as the content of PANICX in the extrudates was increased, except for that with 50 wt.% of PANICX. For simplicity, only SEM micrographs of the etched surfaces of extrudates of blends 10PANICX/SBS and 50PANICX/SBS are presented, which show the effect of the extrusion process on the morphology of the PANICX phase. The presence of elongated cavities formed by the removal of the PANICX phase is shown clearly in Fig. 4, which corresponds to the etched surface of an extrudate of blend 10PANICX/SBS. Figure 4c gives an indication about the possible diameter of a typical elongated cavity. Assuming that the elongated cavities are in the form of longitudinally sectioned microtubes (as exemplified in the model presented in Fig. 5), and that they were created by the PANICX phase that was originally in the form of elongated continuous structures, an average diameter for these PANICX structures of about 4 mm can be estimated.
The presence of the elongated cavities ("the footprints of PANICX"), aligned parallel to the extrusion direction was more evident in extrudates of blends with contents of PANICX up to 35 wt.%, in which a much higher content was observed. This fact seems to be consistent with the content of PANICX in the extruded blends. Very different morphologies to those previously described were observed on the etched surfaces of the extrudates of blend 50PANICX/SBS (Fig. 6). The typical elongated "trails" of PANICX oriented in the extrusion direction were not identified. Indeed, a very irregular surface was observed (e.g. cracks and different types of morphological features) that may have resulted from irregular flow of the melt, taking place in the capillary die entry region or, perhaps, even inside the capillary die itself. The morphologies observed in Fig. 6 thus corroborate the phenomenon of melt fracture that occurred in the flow of the melt during the extrusion of blend 50PANICX/SBS referred to before. Moreover, these morphologies also suggest that the PANICX phase could co-exist in this particular extrudate as both randomly dispersed aggregates and oriented structures, respectively.
The micrographs corresponding to fig 4 evince the formation of PANICX structures oriented in the extrusion direction. Although the structures themselves were not seen, signs of their existence in the extruded blends were evident. The formation of elongated structures of PANICX oriented in the extrusion direction is thus ascribed to a combination of factors, the main ones including:
- The type of flow field occurring during processing. The convergent flow at the entry of the capillary die should have generated a strong elongational flow that drew the PANICX domains into elongated structures. It is also possible that the shear flow taking place inside the capillary contributed to generate elongated structures.
- The viscosity degree of the SBS. The apparent shear viscosity of the SBS matrix is considerably higher than that of the PANI complex. Therefore, it is possible that significant stresses were transferred from the matrix to the dispersed PANICX phase, thereby aiding the drawing process.
- The content of PANICX in the blends. Except for the blend 50PANICX/SBS, it was observed that the formation of elongated structures of the PANI complex was most evident in the extruded blends with higher PANI content. As discussed before, irregular morphological features were found in the specimens of the extrudate 50PANICX/SBS, the main reason being the occurrence of a melt fracture phenomenon in the melt during processing, associated with a lower degree of homogeneity.
3.3. Conductivity measurements
Except for the specimens corresponding to the extrudates of PANICX and 50PANICX/SBS, all the specimens showed resistance levels above the maximum reading achievable by the electrometer (i.e., 200 GW). The results are presented in Table 4. As can be realized, the level of conductivity of the extrudate 50PANICX/SBS was considerably lower than that of the extruded PANICX. Initially, it was believed that the decay in the conductivity was due to a negative effect on the conductivity of the PANICX by subjecting it to a heating-cooling-heating cycle (i.e., blending for compounding, cooling, and blends' extrusion). This could be possible, especially if the number of cycles is very high. However, as explained in sections 2.1 and 2.2, this was not the case. It is very likely that the abrupt decrease in the conductivity of the extrudates, relative to that of the extruded PANICX, is due to a lack of continuity in the PANI complex structures produced within the SBS matrix, associated to inhomogeneities-related problems. This suggests that the process to obtain the extrudates needs to be optimized. In this regard, other investigators [21-24] explain the conduction by means of the formation of conducting paths within the insulating matrix, with or without gaps. They attribute the conduction across the gaps to processes like electron tunneling, which can be understood as the passage of electrons through a potential barrier such as a thin layer of an insulating polymer matrix between two conductors, providing that there are high electric field strengths between the structures of the conducting filler within the insulating matrix. It is evident that the level of conductivity will also depend on the content of such conducting structures, the higher the content, the higher the conductivity because it promotes an increase in the formation of continuous conducting structures. The continuity and amount of such structures will also depend, among many other parameters; upon how homogenous the polyaniline-based blends are.
4. CONCLUSIONS
The PANICX/SBS blends were not completely homogeneous, which is attributed to the fact that the batches used in the preparation of the blends did not have the optimum size. The experiment was not repeated using the optimum batch sizes because previous trials risked the safety and well-functioning of the mixing instrument. That is, batches of the same size were used regardless of the blends composition. As a result, the shearing levels occurring in the melt required to disperse fully the conductive complex within the SBS matrix were not adequate. The formation PANICX structures oriented in the extrusion direction may be ascribed to a combination of factors including elongational and shearing flows occurring in the molten blends during the extrusion process, and to the stresses transferred from the SBS to the PANICX phase. The level of conductivity of the extrudates PANICX/SBS was considerably lower than that of the extruded PANICX. It is thought that the abrupt decrease in the conductivity is due to a lack of continuity in the PANI complex structures, associated to inhomogeneities-related problems.
5. FUTURE WORK
To improve the experiment, a mixing instrument with a higher operational safety limit should be used in order to obtain batches with the optimum size.
REFERENCES
[1] Restrepo, H. F., Cervera, J. G. and Hoyos, B., A. Poly-aniline synthesis by electrochemical oxidation, Dyna-Colombia, 147, pp. 57-63, 2005. [ Links ]
[2] Leyva, M. E., Barra, G. M. O. and Soares, B. G., Conductive polyaniline-SBS blends prepared in solution. Synthetic Met, 123, pp. 443-449, 2001. [ Links ]
[3] Raupp, M. J., Santos, M. F. and Lenz, D. M., Polyaniline Synthesized with Functionalized Sulfonic Acids for Blends Manufacture Materials Research, 10, pp. 425-429, 2007. [ Links ]
[4] Dalas, E., Reparation of dry cells using polypyrrole and polyaniline composites, J. Mater. Sci, 27, pp. 453-457, 1992. [ Links ]
[5] Wen-Chin, C., Jin-Lin, H. and Sung-Nung, L., Synthesis and studies of the physical properties of polyaniline and polyurethane-modified epoxy composites Polym. Eng. Sci., 48, pp. 345-354, 2008. [ Links ]
[6] Harlev, E., Gulakhmedova, T., Rubinovich, I. and Aizenshtein, G., A New method for the preparation of conductive polyaniline solutions: application to liquid crystal devices, Adv. Mater., 8, pp. 994-997, 1996. [ Links ]
[7] Wu, C. Y. and Benatar, A., Microwave welding of high density polyethylene using intrinsically conductive polyaniline, Polym. Eng. Sci., 37, pp. 738-743, 1997. [ Links ]
[8] Elsenbaumer, R. L., Lu, W. K. and Wessling, B., Corrosion protection of mild steel by coatings containing polyaniline, Synthetic Met., 71, pp. 2163-2166, 1995. [ Links ]
[9] Varesano, A., Aluigi, A., Florio, L. and Fabris, R., Multifunctional cotton fabrics, Synthetic Met., 159, pp. 1082-1089, 2009. [ Links ]
[10] Amado, F. D. R., Rodrigues, L. F., Forte, M. M. C. and Ferreira, C. A., Properties evaluation of the membranes synthesized with castor oil polyurethane and polyaniline. Polym. Eng. Sci., 46, pp. 1485-1489, 2006. [ Links ]
[11] Wildi, R. H. and Maier, C., Understanding Compounding, Hanser Publishers, Munich, 1998. [ Links ]
[12] Gazotti, W. A. Jr. and De Paoli, M. A., High yield preparation of poly(o-methoxy aniline) doped with functionalized acids, Synthetic Met., 80, pp. 263-269, 1996. [ Links ]
[13] Yang, C. Y., Cao, Y., Smith, P. and Heeger, A. J., Morphology of conductive, solution-processed blends of polyaniline and poly(methyl methacrylate), Synthetic Met., 53, pp. 293-301, 1993. [ Links ]
[14] Tiitu, M., Talo, A., Forsen, O. and Ikkala, O. T., Aminic Epoxy Resin Hardeners as Reactive Solvents for Conjugated Polymers: Polyaniline Base/Epoxy Composites for Anticorrosion Coatings, Polymer, 46, pp. 6855-6861, 2005. [ Links ]
[15] Cruz, R. H., In-situ Production of Electrically Conductive Polyaniline Fibres from Polymer Blends [PhD Thesis]. Uxbridge, Middlesex, United Kingdom: Brunel University, 2002. [ Links ]
[16] Cruz, R. H., On the characterization of an electrically conductive polyaniline complex, J. Mater. Sci. 39, pp. 511-518, 2004. [ Links ]
[17] KratonTM Polymers Europe/Africa. KratonTM D-1102CU Polymer Data Document. Available: www.kraton.com [citado 7 de Febrero de 2001] [ Links ].
[18] Cruz, R. H. and Cupul, C. V., Structure formation in polyaniline-based polymer blends, J. Mater. Sci., 40, pp. 6571-6579, 2005. [ Links ]
[19] Blythe, A. R., Electrical resistivity measurements of polymer materials, Polym. Test., 4, pp. 195-209, 1984. [ Links ]
[20] Joon, T., Byung, R. and Suck, L., Rheological characterization for the gelation process of polyaniline-emeraldine base/NMP solution by oscillatory test, Korea-Aust. Rheol. J., 22, pp. 273-278, 2010. [ Links ]
[21] Priya, L. and Manjula, G., HCl doped PVA/PAni blends: DC conductivity studies, Adv. Appl. Sci. Res., 3, pp. 489-494, 2012. [ Links ]
[22] Rajagopal, C. and Satyam, M., Studies on electrical conductivity of insulator-conductor composites, J. Appl. Phys., 49, pp. 5536-5542, 1978. [ Links ]
[23] Bridge, B., Folkes, M. J. and Jahankhani, H., Electrical conduction phenomena between adjacent stainless steel fibres in a thermoplastic matrix, J. Mater. Sci., 23, pp. 1955-1960, 1988. [ Links ]
[24] Bridge, B., Folkes, M. J. and Jahankhani, H., Microstructural effects on the charge transfer between non-contacting stainless steel fibres in a polypropylene matrix, J. Mater. Sci. Lett., 7, pp. 1112-1115, 1988. [ Links ]