Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.80 no.180 Medellín jul./ago. 2013
SIMULTANEOUS SACCHARIFICATION AND FERMENTATION OF CASSAVA STEMS
SACARIFICACIÓN Y FERMENTACIÓN SILMUTÁNEA DE TALLOS DE YUCA
HADER CASTAÑO PELÁEZ
MSc. Profesor, Politécnico Colombiano Jaime Isaza Cadavid. Medellin, Colombia. hicastano@elpoli.edu.co
JUAN REALES ALFARO
MSc. Profesor Universidad Popular del Cesar, Colombia. juguireal@gmail.com
JOSÉ ZAPATA MONTOYA
PhD. Profesor Universidad de Antioquia, Medellín, Colombia. jedgar_4@yahoo.es
Received for review November 24th, 2011, accepted March 15th, 2013, final version March, 21th, 2013
RESUMEN: La investigación evalúa el efecto del tamaño de inóculo y la actividad enzimática sobre la concentración de etanol obtenido a través de la estrategia de proceso Sacarificación y fermentación simultáneas de tallos de yuca pretratados con álcalis. La determinación y validación de las condiciones óptimas de producción de etanol y la evaluación del proceso en biorreactor fueron también objeto de esta investigación. Tallos de yuca con pretratamiento alcalino fueron utilizados como sustrato en una relación sólido: líquido 1:10; el complejo enzimático Accellerase 1500 y la levadura Ethanol Red fueron evaluados a dos niveles a una temperatura de 38 ° C y pH 4.0 a escala de erlenmeyer. Se evaluaron como controles del proceso: Sacarificación fermentación simultáneas sin pretratamiento de los tallos y Sacarificación fermentación independientes de tallos pretratados. Se realizó un análisis de regresión y el modelo obtenido fue maximizado empleando algoritmos genéticos. A las condiciones óptimas identificadas en erlenmeyer fue evaluada la producción de etanol en biorreactor de 5 litros. Se obtuvo una concentración experimental de etanol de 1.88±0.04 %v/v (1.99 %v/v óptimo simulado) con una concentración de inóculo de 1.59 g/L y una concentración de enzima de 13.3 FPU/g, valor aproximadamente 4 veces mayor a la cantidad de etanol producido sin pretratamiento por sacarificación y fermentación independientes de tallos de yuca pretratados. La evaluación del proceso en biorreactor alcanzo una concentración de etanol 20% inferior a la alcanzada a escala de erlenmeyer.
PALABRAS CLAVE: Tallos de yuca, Sacarificación, Fermentación, Etanol, Optimización
ABSTRACT: This research evaluates the effects of the inoculum size and enzymatic activity on the concentration of ethanol obtained through the simultaneous saccharification and fermentation of alkali-pretreated cassava stems. Other goals for this study include the determination and validation of the optimal conditions for and the evaluation of the process of ethanol production in a bioreactor. Alkaline-pretreated cassava stems were used as the substrate in a solid to liquid ratio of 1:10; the enzymatic complex Accellerase 1500 and the yeast Ethanol Red were evaluated at two levels at a temperature of 38° C and a pH of 4.0 in an Erlenmeyer flask. The following were evaluated as process controls: simultaneous saccharification and fermentation of non-pretreated stems and separate saccharification and fermentation of pretreated stems. A regression analysis was conducted, and the resulting model was maximized using genetic algorithms. At the optimal conditions identified in an Erlenmeyer flask, the production of ethanol in a 5-liter bioreactor was subsequently evaluated. An experimental concentration of ethanol of 1.88±0.04% v/v (1.99% v/v simulated optimum) was obtained using an inoculum concentration of 1.59 g/L and an enzyme concentration of 13.3 FPU/g. This value was approximately four times the quantity of ethanol produced without pretreatment or by the separate saccharification and fermentation of pretreated cassava stems. The evaluation of the process in the bioreactor yielded an ethanol concentration 20% less than that reached in the Erlenmeyer flask.
KEYWORDS: Cassava Stems, Saccharification, Fermentation, Ethanol, Optimization
1. INTRODUCTION
The threat of depleted oil supplies and environmental concerns have generated interest in biofuels, the production of which has increased dramatically in recent years [1,2].
Ethanol has traditionally been produced using starch and sugary materials [3]; however, given the high cost of these materials and their importance in the production of food and animal fodder, lignocellulosic materials have become interesting and attractive raw materials for the production of ethanol, due to their low cost and abundance [2,4,5].
Lignocellulosic materials are widely available throughout the world at low cost, and this source should be considered for ethanol production with as a way of avoiding competition with the food and agriculture sector.
Cassava stems are one source of agricultural residues that could be considered for bioconversion in tropical countries [6,7]. Potential applications of these materials include activated carbon production, energy generation and animal feed; however, the cassava stems are often left in the field, due to their low monetary value, or are burned, causing environmental problems. Cassava stems can be considered to be an alternative source for the production of bioethanol, and the effects associated with leftover cassava could be mitigated [7-9].
Given that the conversion of lignocellulosic biomass into ethanol is difficult because of the complex structure of the plant cell wall, prior treatment is necessary to alter the structural and chemical composition of the lignocellulosic biomass to facilitate rapid and efficient hydrolysis of the carbohydrates into fermentable sugars [10]. Among the pretreatment methods, alkaline pretreatment has generally been used most frequently because it is more efficient for agricultural residues and herbaceous crops [11].
Various studies report that the products of saccharification hinder the complete conversion of cellulose in lignocellulosic materials [12]. Among the cellulose-based ethanol production systems, simultaneous saccharification and fermentation (SSF) has attracted many researchers [12-14]. The SSF process provides several advantages, such as a greater yield in the production of ethanol because the inhibitory compounds released during saccharification are reduced and also because this method eliminates the need for using separate reactors for saccharification and fermentation and reduces inhibitory processes [12].
Using cassava Colombia has an alternative source of biomass for producing bioethanol. In 2009, the production of this tuber was 1,984,427 tons from an area of 182,313 hectares [15].
To date, there are no records in Colombia concerning the use of agricultural residues from cassava crops for the production of ethanol. Implementing comprehensive, cassava-based production of ethanol (using tubers and stems) would enhance the productivity of the ethanol production process, a reality that is reflected in the increase in the energy index of the process.
Evaluations of cassava-based ethanol production have produced energy index values between 1.34 and 1.43; the use of crop co-products for cogeneration processes or for the production of second-generation ethanol would allow an increase in the energy index [15, 16].
In theory, it is possible to increase the production of ethanol by 25% through the comprehensive use of the cassava crop. Based on the distribution percentages of the cassava crop biomass (50% tuber, 40% stems and 10% foliage) and the country's production of the tuber [17], 1.6 million tons of cassava stems are currently produced, a quantity of biomass that is potentially significant for the production of second-generation ethanol.
The aim of this study was to evaluate the effect of inoculum size and enzymatic load on the concentration of ethanol in a simultaneous saccharification and fermentation process for cassava stems pre-treated using the alkaline method. Finally, we also aimed to determine and validate the optimal ethanol production conditions and evaluate the process within a bioreactor.
2. MATERIALS AND METHODS
2.1. Lignocellulosic Material
Cassava stems of the Copiblanca variety harvested in the Urabá Antioqueño region were dried in an oven at 65°C. The stems were subsequently subjected to manual grinding and grinding via a cutting mill until reaching a particle size of less than 1.4 mm. The pretreated cassava stem flour was obtained by alkaline hydrolysis under the following conditions: NaOH 2.0% solution w/v at 60°C at a 1:10 solid/liquid ratio for 11 hours [18]. The cellulose, hemicellulose and lignin in the remaining solid fraction after pretreatment were characterized.
2.2. Enzymes
The enzymatic complex Accellerase 1500, from Genencor Inc. (Genencor International, Palo Alto, CA, generously donated by Merquiand, Medellín, Colombia) was used, which was derived from a genetically modified stock of Trichoderma reesei, composed mainly of exoglucanase, endoglucanase, hemicellulase and b-glucosidase with an activity of 57.9 FPU/mL.
2.3. Simultaneous Saccharification and Fermentation (SSF) pretreated cassava stems
The simultaneous saccharification and fermentation assays were conducted in 200 mL flasks with a 1:10 solid-solvent ratio. The moisture content of the stems was considered using mass scales. A buffer solution of 0.1 M citrate was prepared with a pH of 4.0 and was mixed with 0.125 g/L MgSO4.7H2O, 1.1 g/L KH2PO4 and 5 g/L yeast extract into a working volume of 70 mL. Of this volume, 20 mL was prepared and enriched with glucose (30 g/L). The 50 and 20 mL volumes were stored in flasks and sterilized at 121°C for 20 minutes.
Following sterilization and cooling of the flasks containing 20 mL of medium to room temperature, corresponding quantities of the yeast Saccharomyces cerevisiae were added, with the reference being powdered Ethanol Red. The experimental design of the Central Design methodology was followed, composed of two evaluated factors at two levels with four repetitions on the central point. The flasks were incubated at 38°C to activate the yeast and maintained in an orbital agitator at 120 rpm for 3 hours.
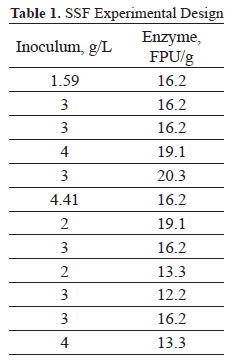
Under aseptic conditions, the 50 mL of medium contained in the flasks and the pre-treated cassava stem flour biomass were mixed. To this mixture, 20 mL of medium containing the activated yeast were added. The experimental units were incubated for 91 hours at 38°C and 120 rpm. Plate counts were performed to evaluate the yeast variability and to observe the presence of contaminating organisms. The quantities of the enzymatic complex and the yeast used in the experimental design are detailed in Table 1. The final concentration of ethanol was considered a response variable for the analysis of the process.
A similar process was undertaken for the control: SSF systems containing cassava stem flour without pretreatment. Three repetitions were performed for the control.
A quadratic model was obtained that relates the response variable to the significant effects. This model was used to optimize the response variable in the evaluation interval of the factors. The STATGRAPHICS Centurion XVI software was used to perform the statistical analysis. The maximum value of the function was obtained using the genetic algorithm optimization tool in Matlab R2008b with 100 generations, a reproduction factor of 0.8, an initial population of 20 and an error of 1e-6 as the stopping criterion. Experimental validation was conducted on the optimal conditions determined (three repetitions).
2.4. Separate Saccharification and Fermentation (SHF)
The SHF process for pre-treated cassava stems was carried out for 60 hours at 50°C in a 0.1 M citrate/citric acid buffer solution at a pH of 5.0, in a solid/solvent relationship of 1:10. The dose of enzymatic complex used was that which generated the best results in the SSF process. Following the saccharification process, the enzyme was inactivated by subjecting the medium to 90°C for 5 minutes. Subsequently, the medium was enriched with 0.125 g/L MgSO4.7H2O, 1.1 g/L KH2PO4 and 5 g/L yeast extract, and the pH was adjusted to 4.0 using a buffer solution of 0.1 M citric acid. The system was sterilized at 121°C for 20 minutes. Once the system cooled to room temperature, the yeast was activated in the same manner as in the SSF process. After activating the yeast, the system was inoculated with the quantity of yeast that yielded the best results in the SSF process and incubated at 38°C and 120 rpm for 31 hours. Three repetitions of the SHF process were performed, and plate counts were also performed to evaluate viability of the yeast and examine any contamination.
2.5. Simultaneous Saccharification and Fermentation in 5-liter bioreactor
The SSF process was carried out in a 5 L bioreactor (3 L of usable volume) in which the production of ethanol was evaluated. The following conditions were used: inoculum size of 1.59 g/L, pH of 4.0, 38°C, 8.33 Hz, an enzymatic complex load 13.3 FPU/g of solids and a pre-treated cassava stem flour load/solvent ratio of 1:10. Two repetitions of the process were conducted, and the temperature, pH and frequency were controlled.
2.6. Analytic Methods
The cellulose, hemicellulose and lignin contents in the bulk biomass and in the pretreated cassava stems were calculated according to the methods described by Van Soest [19]. The enzymatic activity of the complex was determined according to the procedure recommended by the International Union of Pure and Applied Chemistry (IUPAC), which defines a filter paper unit as the quantity of enzyme that releases 1 micromole of glucose (reducing sugars) per minute under assay conditions [20]. The determination of the glucose and ethanol was conducted via high-performance liquid chromatography (HPLC). The HPLC system used a BIORAD Aminex HPX-87H column. The mobile phase used was 0.008 N H2SO4 with a flow volume of 0.5 mL/min. A Refraction Index system was used for detection. The temperature of the 20 mL injection volume was maintained at 30°C with an oven. The concentrations were determined in triplicate.
3. RESULTS AND DISCUSSION
3.1. Pretreatment of cassava stems
Three delignification strategies were evaluated in a previous study aimed to improve or facilitate the accessibility of the enzyme to the lignocellulosic matrix of cassava stems [18]. Of the delignification methods evaluated (acid hydrolysis, alkaline hydrolysis and organosolvent with ethanol), alkaline hydrolysis resulted in the greatest percentage of lignin removal (36.35+/-5.50%). The maximum lignin removal occurred at a concentration of 2.0% w/v of NaOH at 60°C for 11 hours.
The cellulose contents of the cassava stem biomass were similar to that reported by other researchers [6,7,17], although there was a difference in the lignin and hemicellulose contents. This difference can be explained by the different varieties used in the studies and uncertainty regarding the vegetative state when the plant was harvested.
Based on the composition of the cassava stems before and after the alkaline hydrolysis reported in Table 2, 44.24% of the lignin was eliminated, and 78.54% of the cellulose and 89.5% of the hemicellulose from the cassava stems were retained after pretreatment. These are important data for the valuation of the transformation of residues into ethanol because there is a high recovery of fermentable sugar source polymers.
Studies on pretreatments report that changes in the concentration of NaOH have a significant effect on the removal of lignin from different lignocellulosic materials [21-23]. In the literature, greater percentages of lignin removal have been reported than those achieved in this study, which can be attributed to the use of NaOH concentrations higher than 10% w/v and to the different nature of the substrate (cotton stems and corn cobs).
3.2. Simultaneous Saccharification and Fermentation
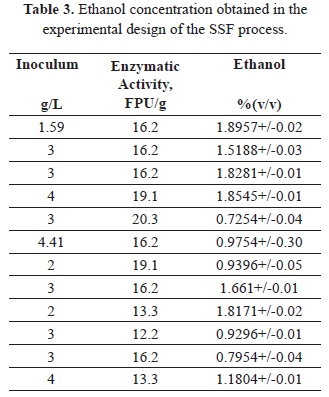
Table 3 presents the concentrations of ethanol obtained through the SSF process under the experimental and control conditions. The ethanol concentration varied between 0.725 and 1.896% v/v.
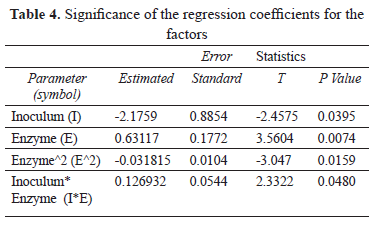
The results of the multiple regression analysis are presented in Table 4.
The positive influence of the enzyme in its linear term is explained by the nature of the simultaneous process, in which the response variable is a consequence of the coupling of two consecutive biological processes and is controlled by enzymatic saccharification.
The positive effect of the inoculum/enzymatic activity interaction is supported by the need to find an adequate relationship that permits the balancing of the two biological reactions. This balancing allows the speed of glucose release to sustain the energy requirements of the yeast's metabolism.
The negative effect of the inoculum size on response variable in the SSF process is explained by the fact that under the conditions evaluated, the rate at which fermentable sugars are released makes it difficult to support the needs of the yeast substrate. This effect is validated by the experimental observation that the greatest concentration of ethanol (1.89% v/v) was reached when the lowest level of inoculum was detected (1.59 g/L).
The Regression Analysis model returned an R2 of 0.95, with a P statistic value of less than 0.05, which indicates that the model is adjusted and that it explains 95% of the variation in the percentage of ethanol produced during the process.
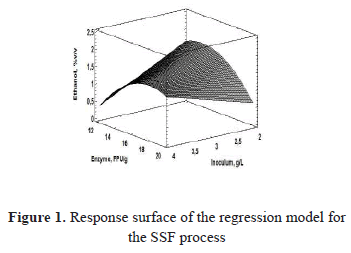
Figure 1 shows the non-linearity between the response variable and the factors analyzed, and the maximum in the intervals of the evaluated levels is presented.
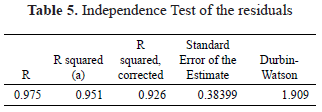
The evaluation of the residuals of the model via the Durbin-Watson statistic (1.9) confirms the hypothesis of independence of the residuals. According to the observations presented in Table 5, the model presents a corrected R2 of 0.926, indicating a good fit of the model to the experimental data.
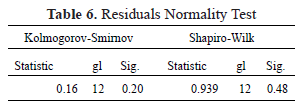
The Normality tests using the Shapiro-Wilk and Kolmogorov-Smirnov statistics shown in Table 6 with significance values of 0.48 and 0.20 allow the validation of the hypothesis of the normal distribution of the residuals, demonstrating the goodness of fit of the regression model.
3.3. Optimization of the SSF process
After maximizing the concentration of ethanol using genetic algorithms (after 51 iterations), an ethanol concentration of 1.99% v/v was estimated with an enzymatic activity of 13.3 FPU/g of biomass and an inoculum concentration of 1.59 g/L. The experimental validation of these conditions achieved a concentration of 1.883 +/- 0.04% v/v (14.7 g/L) ethanol after 91 hours of processing. Han et al. (2011) [10] obtained an ethanol concentration of 0.9679% v/v using cassava stems pretreated with acid and separate saccharification and fermentation.
The SSF process resulted in the conversion of 0.23 g of ethanol/g AR. When conducting the SSF process without pretreatment as a control, 0.51 +/- 0.02% v/v of ethanol was obtained. The production of ethanol using the SSF pretreatment strategy was 4 times the concentration obtained without prior treatment of the biomass, which demonstrates the importance of pre-treatment in order to improve the enzymatic digestibility of cassava stems. A similar result was obtained by Han et al. [10] using acid pretreatment of cassava stems. Fernández et al. (2008) [2] evaluated the fermentability of the acid prehydrolyzed biomass of cassava stems and obtained a similar yield to that found in this study (0.23 g ethanol/g total sugar), which improved slightly at a dilution of 50% (0.29 g ethanol/g total sugar).
3.4 Separate Saccharification and Fermentation
The process of separate saccharification (60 hours) and fermentation (31 hours) of cassava stems generated a concentration of 0.52 +/-0.03% v/v, which was a much lower value compared to the SSF process (1.88% v/v). This demonstrates the advantage of the SSF strategy, given that, when the processes were conducted separately, more enzyme inhibition occurs, possibly as a result of glucose accumulation, which inhibits cellobioses, which remain as cellobiose and do not hydrolyze.
Han et al. [10] obtained a greater ethanol concentration (0.97% v/v) from cassava stems using separate saccharification and fermentation processes under optimized conditions with acidic prehydrolyzed biomass. The difference in the ethanol yield can be explained by the different effects of the acid and alkaline pretreatments on the lignocellulosic matrix of the cassava stems and the addition of the enzyme b glucosidase, which facilitates cellobiose hydrolysis and improves the cassava stems fermentability.
The concentration of ethanol obtained from the SSF process without biomass pretreatment was similar to that obtained in the SHF process with biomass pretreatment. Martin et al. [7] obtained reduced enzymatic convertibility in the pretreated material compared to the non-pretreated material, indicating that the non-sugar products of hydrolysis can have inhibitory effects or can affect the speed of the enzymatic reaction. The concentration of ethanol in the SSF process was greater than that obtained in the SHF process (1.88% vs. 0.51% v/v), demonstrating the advantage of integrating the stages of saccharification and fermentation to reduce the glucose-mediated inhibition of the enzymatic complex.
The ethanol yields obtained using hydrolyzed cassava stem biomass are greater than 80% [10,18,25,26], but despite these high ethanol yields using glucose, the concentrations of ethanol achieved (5.42-15.7 g/L) are low for a technically and economically viable process. Thus, it is necessary to pursue research into other types of pretreatments and the integration of simultaneous stages of saccharification and fermentation.
3.4 SSF in the 5-liter bioreactor
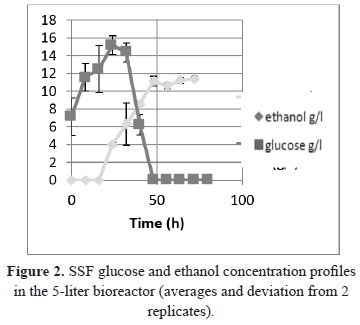
Two replicates of the SSF process were performed in a 5-liter bioreactor with a usable volume of 3 L. The frequency of agitation required to keep the system homogeneous was 8.33 Hz, and all other operating conditions were the same as the optimal conditions described in the flask method. As observed in Figure 2, the glucose and ethanol concentration profiles were similar for both replicates of the process.
The concentration of ethanol after 72 hours of processing (11.59 g/L; 1.48% v/v) was 80% of the concentration obtained through the process in the Erlenmeyer flask, and this result can be explained by the change in scale, owing to the changes in the kinetic parameters of momentum and mass between the two scales (250 mL Erlenmeyer flask with a volume of 100 mL agitated orbitally vs. a bioreactor with a usable volume of 3 L with 500 rpm) and the different configurations of the mixture. It should also be mentioned that the enzymatic/fermentative system consists of three phases (solid: pretreated biomass, liquid: dispersion medium, gas: liberation of carbon dioxide), which is an aspect that complicates the phenomenological analysis because problems with homogeneity and dispersion were observed with the evaluated solid load.
4. CONCLUSIONS
The SSF process strategy resulted in a reduction of the processing times and a generation of a greater concentration of ethanol (1.88% v/v for SSF vs. 0.52% v/v for SHF). Biomass pretreatment allows a greater concentration of ethanol to be obtained using the SSF strategy (1.88% v/v vs. pretreatment 0.51% v/v without pretreatment).
Under the conditions evaluated, the enzymatic activity and the enzymatic activity-inoculum interaction resulted in positive effects on the production of ethanol, while the inoculum and the quadratic term of the enzymatic activity resulted in a negative effect.
The optimal conditions validation of the inoculum and enzymatic activity generated ethanol at a concentration of 1.88% v/v (14.7 g/L).
In the evaluation of the scale change to a 5 L bioreactor that was agitated mechanically, a 20% reduction in the final ethanol concentration was observed after 72 hours of processing (11.5 vs. 14.7 g/L). Due to the coexistence of three phases involved in the SSF processes for obtaining second-generation ethanol, the scaling analysis requires a different evaluation with respect to the traditional scaling used in first-generation ethanol production processes.
5. ACKNOWLEGMENTS
The authors are grateful to Politécnico Colombiano Jaime Isaza Cadavid for financing the project 2061100144 and Universidad de Antioquia for the sustainability strategy.
REFERENCES
[1] Mabee, W. and Saddler, J., Bioethanol from lignocellulosics: Status and Perspectives in Canada, Bioresource Technology., 101, pp. 4806-4813, 2010. [ Links ]
[2] Fernández, T., Martín, C., Marcet, M. and Thomsen, A.B., Fermentability of prehydrolyzed lignocellulosic residues for the production of ethanol, Chemical Engineering., 40 (455), pp. 196-197, 2008. [ Links ]
[3] Krishna, S. and Chowdary, G., Optimization of simultaneous saccharification and fermentation for the production of ethanol lignocellulosic biomass, J. Agric. Food Chem., 48, pp. 1971-1976, 2000. [ Links ]
[4] Sánchez, O. and Cardona, C., Trend in biotechnological production of fuel ethanol from different feedstock, Bioresource Technology., 99, pp. 5270-5295, 2008. [ Links ]
[5] Howard, R.L., Abotsi, E., Jansen, E.L. and Howard, S., Lignocellulose biotechnology: issues of bioconversion and enzyme production, African Journal of Biotechnology., 2 (12), pp. 602-619, 2003. [ Links ]
[6] Martín, C., López, Y., Plasencia, Y. and Hernández, E., Characterization of agricultural and agro-industrial residues as raw materials for ethanol production, Chem. Biochem. Eng. Q., 20 (4), pp. 443-447, 2006. [ Links ]
[7] Martín, C., Alriksson, B., Sjöde, A., Nilvebrant, N. and Jönsson, L., Dilute Sulfuric Acid Pretreatment of Agricultural and Agro-Industrial Residues for Ethanol Production, Applied Biochemistry and Biotechnology., 137-140 ( 1-12), pp. 339-352, 2007. [ Links ]
[8] Balat, M.. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review, Energy Conversion and Management., 52 (2), pp. 858-875, 2011. [ Links ]
[9] Pandey, A., Soccol, C., Nigam, P., Soccol, V., Vandenberghe, L. and Mohan, R., Biotechnological potential of agro industrial residues. II: cassava bagasse, Bioresource Technology., 74, pp. 81-87, 2000. [ Links ]
[10] Han, M., Kim, Y., Chung, B. and Choi, G., Bioethanol production from optimized pretreatment of cassava stem, Korean J. Chem, Eng., 28 (1), pp. 119-125, 2011. [ Links ]
[11] Sun, Y. and Cheng, J., Hydrolysis of lignocellulosic materials for ethanol production. A review, Bioresource Technology., 83 (1), pp. 1-11, 2002. [ Links ]
[12] Li, H., Kim, N., Jiang, M., Kang, J. and Chang, H., Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid-acetone for bioethanol production, Bioresource Technology., 100, pp. 3245-3251, 2009. [ Links ]
[13] Zyl, J., Rensburg, E., Zyl, W., Harms, T. and Lynd, L., A kinetic model for simultaneous saccharification and fermentation of Avicel with Saccharomyces cerevisiae, Biotechnology and Bioengineering., 108, pp. 924-933, 2011. [ Links ]
[14] Rodríguez, R., Gernaev, K., Meyer, A. and Sin, G., A mathematical model for simultaneous saccharification and co-fermentation of C6 and C5 sugars, Chinese Journal of Chemical Engineering., 19 (2), pp. 185-191, 2011. [ Links ]
[15] Castaño, H., Naranjo, C. and Botero, J., Life Cycle Assessment for Bioethanol production from Cassava in Colombia, Report from the IV International Conference on Life Cycle Analysis, Coatzacoalcos, México, pp. 35-39, April 2011. [ Links ]
[16] Yu, S. and Toa, J., Energy efficiency assessment by life cycle simulation of cassava-based fuel ethanol for automotive use in Chinese Guangxi context, Energy., 34, (1), pp. 22-33, 2009. [ Links ]
[17] Castaño, H., Cardona, M., Gómez, C. and Acosta, A., Ethanol production from cassava flour in simultaneous enzymatic hydrolysis and fermentation system, Dyna., 78 (109), pp. 158-166, 2011. [ Links ]
[18] Reales, J., Obtaining ethanol using agricultural residues from cassava.[Master's thesis]. Medellin, Antioquia : Universidad de Antioquia, 2012. [ Links ]
[19] Van Soest, P., Use of detergent in the analysis of fibrous feeds. II A rapid method for the determination of fiber and lignin, J Assoc of Anal Chem., 46 (1), pp. 829-835, 1963. [ Links ]
[20] Ghose, T. K., Measurement of cellulase activities, Pure Appl. Chem., 59, pp. 257-263, 1987. [ Links ]
[21] Alvira, P., Tomás-Pejó, E., Ballesteros, M., and Negro, M.J., Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review, Bioresource Technology., 101 (13), pp. 4851-4861, 2010. [ Links ]
[22] Carvalheiro, F., Duarte, L.C. and Gírio, F.M., Hemicellulose biorefineries: a review on biomass pretreatments, J. Sci. Ind. Res., 67 (11), pp. 849-864, 2008. [ Links ]
[23] Silverstein, R., Chen, Y., Sharma-Shivappa, R., Boyette, M. and Osborne, J., A comparison of chemical pretreatment methods for improving saccharification of cotton stalks, Bioresource Technology., 98 (16), pp.3000-3011, 2007. [ Links ]
[24] Varga, E., Scengyel, Z. and Recaey, K., Chemical pretreatments of corn Stover for enhancing enzymatic digestibility, Applied Biochemistry and Biotechnology., 98-100 ( 1-9), pp. 73-87, 2002. [ Links ]
[25] Nuwamanya, E., Chiwona, L., Kawuki, R. and Baguma, Y., Bio-Ethanol Production from Non-Food Parts of Cassava (Manihot esculenta Crantz), Ambio., 41 (3), pp.262-270, 2012 [ Links ]
[26] Sovorawet, B. and Kongkiattikajorn, J., Bioproduction of ethanol in SHF and SSF from cassava stalks, KKU Res. J., 17 (4), pp. 565-572, 2012. [ Links ]