Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.80 no.180 Medellín jul./ago. 2013
KINETICS OF ALCOHOLIC FERMENTATION USING GUAVA (Psidium guajava) SEED FLOUR AND DRY MYCELIUM OF Aspergillus niger AS NITROGEN SOURCES
CINÉTICA DE FERMENTACIÓN ALCOHÓLICA UTILIZANDO COMO FUENTE DE NITRÓGENO HARINA DE SEMILLA DE GUAYABA (Psidium guajava) Y MICELIO SECO DE Aspergillus niger
LILIANA SERNA COCK
Doctora en Ingeniería, Universidad Nacional de Colombia Sede Palmira, lserna@unal.edu.co
JOSÉ DANIEL MERA AYALA
Ingeniero Agroindustrial, Universidad Nacional de Colombia Sede Palmira, jdmeraa@unal.edu.co
JORGE EDUARDO ANGULO LÓPEZ
Ingeniero Agroindustrial, Universidad Nacional de Colombia Sede Palmira, jeangulol@unal.edu.co
ANA LUCIA GÓMEZ SCHOUBEN
Magister en Ciencias biológicas, SUCROAL S.A, algomez@sucromiles.com.co
Received for review December 9th, 2011, accepted March 15th, 2013, final version March, 21th, 2013
ABSTRACT: The kinetics of alcoholic fermentation was evaluated using the following substrates: molasses, urea, and diamine phosphate (MUDP); molasses and guava seed flour (MGSF); and molasses and dry mycelium of Aspergillus niger (MMAN). The fermentations were done using Saccharomyces cerevisiae under anaerobic conditions, 32°C, and pH 4.6. For each fermentation substrate the effect of the nitrogen source on cell viability (V), substrate conversion (CS), ethanol yield (Yp/s), and productivity was evaluated. The MGSF substrate showed the highest product yield (5.86 gg-1) and substrate conversion (47.58%) at 18 and 24 h of fermentation, respectively, with a maximum ethanol concentration of 60 gL-1. Substrate MMAN showed a low ethanol yield (36 gL-1). It was concluded that guava seed flour is a viable and economic nitrogen source for alcoholic fermentations.
KEYWORDS: Co-products, ethanol, Saccharomyces cerevisiae, cellular viability.
RESUMEN: Para la cinética de fermentación alcohólica se evaluaron como sustratos melaza más urea y fosfato diamónico (MUFD), melaza más harina de semilla de guayaba (MHSG) y melaza más micelio seco de Aspergillus niger (MMAN). Las fermentaciones se realizaron anaeróbicamente, a 32°C, pH 4.6 y usando Saccharomyces cerevisiae. Para los sustratos mencionados se estudió el efecto de la fuente de nitrógeno en la viabilidad celular (V), conversión de sustrato (CS), rendimiento en etanol (Yp/s) y productividad. El sustrato MHSG presentó el mayor rendimiento en producto y la mayor conversión de sustrato a las 18 y 24 horas de fermentación respectivamente (5.86 gg-1 y 47.58%), con una concentración máxima de etanol de 60 gL-1. El sustrato MMAN presentó un bajo rendimiento en la producción de etanol (36 gL-1). La harina de semilla de guayaba se puede considerar una fuente de nitrógeno viable y económica en fermentaciones alcohólicas.
PALABRAS CLAVE: Residuos, etanol, Saccharomyces cerevisiae, viabilidad celular.
1. INTRODUCTION
The availability and stability of fermentation substrates are key research topics for the fermentation industry, because they represent up to 68% of the production cost [1, 2]. Carbon and nitrogen are the most important components in raw materials used during fermentation. Sugar cane molasses, one of the main by-products of the sugar industry, is a good source of carbon that does not require complex pre-processing [3]. The main source of nitrogen for industrial fermentations is urea, which is critical for cellular development. In addition, phosphorus (as diamine phosphate) is added because it is easily absorbed by the microbial cells and also favors cellular development [4]. The search for new nutrient sources is directed towards replacing costly supplements (e.g., peptides as nitrogen sources) and important growth factors (e.g., vitamin B complex) [5].
One of the most common problems in the food industry is the elimination of waste products, due to limited disposal sites, processing costs, ecological impact, and the need to generate valuable products from waste materials [6, 7]. The enormous quantities of biomass waste generated in food processing plants can be potentially used to produce biofuels, animal feed, chemical products, and enzymes, among others [8].
During the processing of guava based jellies, sorbets, pastes, and sweet bars, a large quantity of high protein waste products are generated [6, 9, 10]. The discarded seeds represent 12% of the fresh fruit weight and have a high protein content [7]. According to the Regional Unit of Agricultural Planning (URPA), there are 12,500 ha of guava in Santander province (Colombia): 9,500 ha in Vélez and 3,000 ha in Comunera, with an annual yield of ca. 7 to 8 tonne ha-1 [11].
Research is needed to establish whether the waste generated during the processing of guava, can be used as an alternative source of protein with potential applications in industrial fermentation [5, 12, 13]. In recent years efforts were made to increase ethanol production as the most promising biofuel from renewable resources [14].
Another waste generated in the food and pharmaceutical industries is the dry mycelium of Aspergillus niger. The final fermentation biomass contains ca. 20 gL-1 of mycelium with a significant amount of protein that can be used as an alternative source of nitrogen for other fermentations [15, 16].
The purpose of this research was to evaluate the efficiencies of guava seed flour and dry mycelium of Aspergillus niger, as alternative efficient nitrogen sources in alcoholic fermentations. Efficiency was measured in terms of cellular viability, substrate conversion, ethanol yield, and productivity.
2. METODOLOGY
2.1. Biological Material
Guava (Psidium guajava) seeds were supplied by the Dulces San Antonio la Tentación company (Vélez, Santander, Colombia). The seeds were dried using air at 60ºC for 24 h (Binder ED 115-UL, Germany) and then milled to a fine powder (flour) of ca. 0.5 mm diameter (FRITSCH Pulverisette 14, Germany). The dry mycelium of Aspergillus niger was supplied by Sucromiles S.A. (Valle del Cauca, Colombia). The raw materials were characterized by proximate analysis (moisture, nitrogen, protein, ash, ethereal extract, fiber, total and reducing sugars), gross energy, iron, potassium, zinc, magnesium, calcium, phosphorus, vitamin A, vitamin C, vitamin B2, vitamin B3, and vitamin B6.
2.2. Characterization of the biological material
2.2.1. Dry mass and moisture content
The dry mass and moisture content were determined following official method AOAC 934.01; the biological materials were dried at 60ºC and then at 105ºC until constant weight was obtained (Binder ED 115-UL, Germany) [17].
2.2.2. Nitrogen and protein content
The nitrogen content was determined by Kjeldahl [18] and a factor of 6.25 was used for the conversion of nitrogen to protein.
2.2.3. Ash content
Ash was determined following official method AOAC 942.05 using a muffle furnace (Fischer Scientific 550-58, USA) at 600ºC for 6 h [17].
2.2.4. Ethereal extract
The ethereal extract was determined following official method AOAC 920.39. Petroleum ether (reagent grade) was used as the solvent [17] in a Soxhlet extractor (Soxhlet S6-E2, Colombia).
2.2.5. Fiber (hemicellulose, cellulose and lignin) content
Fiber content was determined following the Ankom method, discriminating for hemicellulose, cellulose and lignin present in the biological materials [19].
2.2.6. Total sugars content
Total sugars were determined by spectrophotometry according to the Antrona method [20]. Absorbance was measured at 625 nm (ThermoScientific-Genesys 10UV, USA).
2.2.7. Reducing sugars content
Reducing sugars were determined by spectrophotometry according to the DNS method (Dinitrosalicylic acid) [21]. Absorbance was measured at 575 nm (ThermoScientific -Genesys 10UV, USA).
2.2.8. Gross energy content
The gross energy present in the biological materials was determined by the combustion of the material in a calorimetric pump with oxygen at 30 atm [19].
2.2.9. Iron, potassium, zinc, magnesium, calcium and phosphorus content
Iron, potassium, zinc, magnesium, and calcium were determined by atomic absorption spectroscopy (Perkin Elmer -Analyst 400, USA) and the phosphorus was determined by spectrophotometry UV/VIS (Thermo Electron -Genesys 10UV, USA). Absorbance was measured at 650 nm.
2.2.10. Vitamins A, C, B2, B3 and B6 content
Vitamin content was measured by HPLC (Perkin Elmer - Series 200, USA) using a Shodex C18 column (250 x 4 mm). Vitamin A was measured by absorbance at 325 nm using methanol/water as the mobile phase. Vitamins C, B2, B3 and B6 were measured by absorbance at 271 nm using phosphate buffer at pH 2.8 as the mobile phase.
2.3. Alcoholic fermentation
2.3.1. Microorganism
The Ethanol Red strain of Saccharomyces cerevisiae (Division of S.I. Leaffre Group, France) was utilized.
2.3.2. Fermentation substrate
For the reproduction stage, the following substrates were used: molasses, urea, and diamine phosphate (MUDP, reducing sugars 80 gL-1, urea 1.1 gL-1 and diamine phosphate 0.67 gL-1); molasses and guava seed flour (MGSF; reducing sugars 80 gL-1, guava seed flour 51.42 gL-1); and molasses and dry mycelium of Aspergillus niger (MMAN; reducing sugars 80 gL-1, dry mycelium of Aspergillus niger 19.73 gL-1). For the fermentation stage, molasses (250 gL-1) was used as substrate. The substrates were adjusted to a pH of 4.6 (Mettler Toledo - SevenEasy, Switzerland), using sulfuric acid in water 98% and sterilized at 121°C and 0.101 MPa for 15 min (Autoclave sterilizer Medical - Essen 250 I, Colombia).
2.3.3. Preparation of the inoculum
The initial innoculum was made of 300 mL of substrate and 1 gL-1 of Saccharomyces cerevisiae. In the yeast reproduction stage the substrates remained in a controlled temperature bath (Julabo - 13A, Germany) at 34 ± 0.5°C, with 5 Lmin-1 aeration and stirring at 270 rpm. Initial biomass growing was done for 4 h to yield at least 3x108 CFU mL-1 as suggested by Calderón [3]. Then each substrate was inoculated in molasses (250 gL-1) to start the fermentation stage.
2.3.4. Fermentation Stage
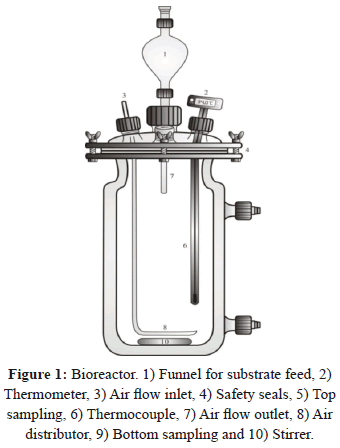
The kinetics of fermentation was studied in 9 fermentation batches, using a 1500 mL bioreactor (Figure 1), with an effective working volume of 1000 mL. During this stage the substrates remained in a controlled temperature bath (Julabo - 13A, Germany) at 32±0.5°C and were stirred at 270 rpm for 24 h. The effective working volume was divided into six equal volumes (116.6 mL each). Every hour the substrate was added to the bioreactor with the above-mentioned volume (the inoculum volume of 300 mL, was added at 0 h) in order to achieve a strain adaptation to the osmotic pressure generated by the sugar concentration in the substrate and to decrease the fermentation time [4].
The fermentation stage was done under anaerobic conditions, by hermetically closing all inlets to the bioreactor and capturing the excess CO2 using a 0.1 N sodium hydroxide solution trap.
2.3.5. Kinetics of substrate use, biomass formation and ethanol yield during fermentation
In order to study the kinetics of substrate consumption and biomass formation during the reproduction stage, 10 mL of each substrate at 0, 1, 2, 3 and 4 h of reproduction were aseptically collected (time 0 corresponded to the addition of yeast), centrifuged at 4000 rpm for 10 min (Eppendorf Centrifuge - 5804R, Germany), and then filtered (0.45 mm pore size; Titan, USA). To determine the kinetics of substrate consumption, biomass formation, and ethanol yield during the fermentation stage, 10 mL of each substrate at 0, 3, 6, 12, 18, and 24 h of fermentation was aseptically collected (time 0 corresponded to the final conditions of the cellular reproduction stage), centrifuged and filtered as described before. The substrate consumption (reducing sugars) was determined by spectrophotometry (ThermoScientific -Genesys 10UV, USA) by the DNS method (Dinitrosalicylic acid) [21]. The kinetics of biomass formation was determined by count in the Neubauer Chamber, in the sample before centrifugation; methylene blue (1% wv-1) was used as the colorant in order to determine the cellular viability [3, 4].
2.3.6. Ethanol content determination
The ethanol production was measured in the supernatants by gas chromatography (7890A GC, Agilent Technologies, USA) [22, 23, 24, 25, 26] with 1 mL injection volume, 180ºC injector temperature, 100:1 split, 0.8 mLmin-1 column flow, 75ºC/8 min column temperature, ZB-Wax column (50 m x 250 mm x 1 mm), 260ºC detector temperature, and helium as a carrier gas. The cellular viability (V) (Equation 1), the substrate conversion (CS) (Equation 2), the product yield (Yp/s) (Equation 3) and the productivity (Equation 4) were calculated as follows:
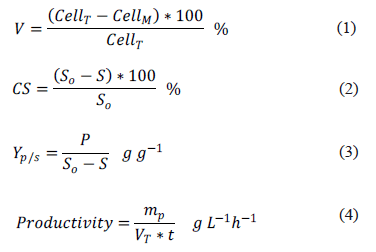
Where, CellT is the number of total cells, CellM is the number of dead cells, S0 is the initial concentration of reducing sugars (gL-1), S is the final concentration of reducing sugars (gL-1) at maximum ethanol concentration, P is the maximum ethanol concentration (gL-1), mp is the mass of ethanol (g), VT the total fermentation volume (L), and t is the fermentation time (h).
2.4. Experimental design and statistical analysis
For the reproduction stage, a factorial design of 3*5 with three replications was used. Factor substrate had 3 levels (MUDP, MGSF, and MMAN), and factor reproduction time had 5 levels (0, 1, 2, 3 and 4 h). The response variables were: cellular viability and substrate conversion. For the fermentation stage, a factorial design of 3*6 with three replications was used. Factor fermentation substrate had 3 levels (MUDP, MGSF, and MMAN). Factor fermentation time had 6 levels (0, 3, 6, 18, and 24 h), where time 0 corresponded to the final conditions of the reproduction stage. The response variables were cellular viability, substrate conversion, product yield and productivity. Results were analyzed using SAS software (version 9.2, SAS Institute Inc., Cary, N.C., USA). Multiple comparison among means was done using the LSD test with P<0.05.
3. RESULTS AND DISCUSSION
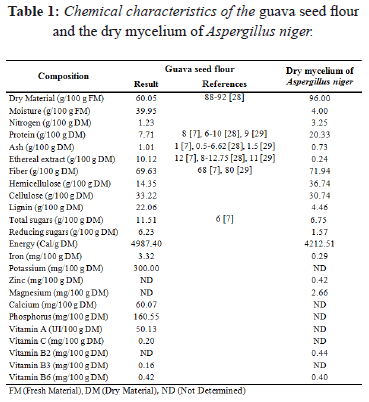
Table 1 shows the chemical composition of the guava seed flour and the dry mycelium of Aspergillus niger. The nitrogen content (dry basis) of the dry mycelium of Aspergillus niger was 2.64 times higher than that of the guava seed flour.
The ethereal extract content in the guava seed flour also proved to be interesting since it had an antifoam effect during the alcoholic fermentation stage. Antifoaming agents are used in industrial fermentation to prevent microbial contamination and substrate losses. Antifoams are traditionally mixed with non-polar hydrophobic oils (silicone oil) or agglomerates to increase the solid particles during the fermentation stage [4, 27]. Guava seed flour, due to its content of ethereal extract, can be an alternative to the use of antifoaming agents, since it contains linoleic acid (79%), palmitic acid (8%), oleic acid (7%), stearic acid (5%), and triglycerides (60% trilinolein) [28], that have antifoaming properties during the fermentation process.
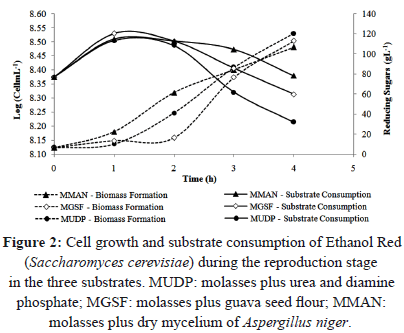
Figure 2 shows that the reducing sugars increased in concentration during the first hour of the reproduction stage, explained by the action of the yeast enzyme invertase breaking sucrose into simple sugars [29]. The hydrolysis of sucrose promoted a slight increase in the concentration of fructose and glucose [30], and therefore an increase in reducing sugars.
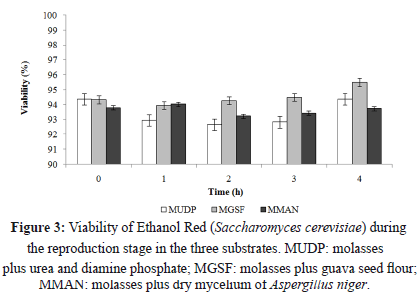
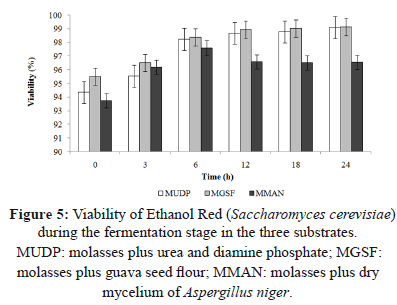
Greater cellular viability during fermentation was observed when the MGSF substrate was used (Figure 3 and 5), probably due to the higher vitamin content (B3 and B6) in guava seeds. The addition of vitamins in the fermentation processes is required for the growth of some microbial strains, because vitamins contribute to cellular respiration, electron transfer, and the production of amino acids used during the stage of cellular growth [31]. Similarly, the high potassium and phosphorus content in the MGSF substrate would favor enzymatic action for the synthesis of nucleic acids and phospholipids. Iron also plays a fundamental role in cellular respiration and represents a key element of cytochromes and proteins involved in electron transfer, generating greater cell viability during fermentation [31]. According to Yegres et al. [32], the addition of dibasic ammonium phosphate (0.2 gL-1), promotes yeast production and development without affecting distillation quality. The phosphorus content was 0.16% in the guava seed flour (Table 1) and 8.23 gL-1 in the MGSF substrate, allowing for an optimum production of alcohol without the need of external phosphate sources.
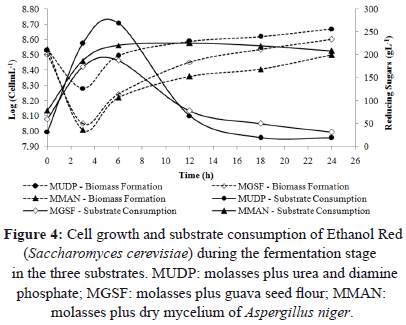
The increase in reducing sugars observed during the first 6 h of the fermentation stage (Figure 4) was explained by the constant substrate feed during this period. The number of viable cells (biomass) was reduced due to dilution, also explained by the substrate feed. At the end of the fermentation stage, there were statistically significant differences (P<0.05) in substrate consumption and biomass formation among the three substrates. Substrates MUDP and MGSF showed a residual substrate of 18.45 and 31.23 gL-1 respectively. Studies carried out by Peña et al. [26] using molasses (250 gL-1) showed similar behavior in substrate consumption. However, they reported final residues with concentration higher than 30 gL-1.
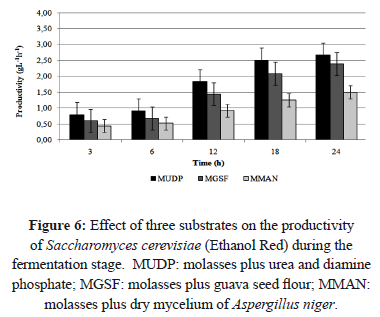
The MGSF substrate showed the highest product yield (Yp/s) and the highest substrate conversion at 18 and 24 h of fermentation, respectively (5.86 gg-1 and 47.58%) (Figure 4). After 24 h of fermentation, the productivity in MUDP substrate was higher than in MGSF substrate, with values of 2.67 and 2.39 gL-1h-1, respectively (Figure 6). In the MGSF substrate the product yield was greatest at 18 h of fermentation, while in the MMAN substrate, the highest productivity was shown at 24 h (1.5 gL-1h-1, 43.66% and 37.24% less than the productivity of the MUDP and MGSF substrates, respectively).
The highest productivity was obtained using substrate MUDP, followed by substrate MGSF. The lowest productivity was obtained using substrate MMAN.
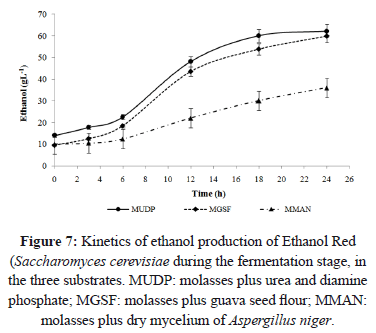
In substrates where no urea or diamine phosphate were used (i.e., substrate MGSF and MMAN), higher ethanol concentration was observed when guava seed flour was used as the nitrogen source (Figure 7). This behavior was probably due to higher nutrient content (e.g., vitamins and minerals) in guava seeds [6, 33, 28, 34]. The low ethanol yield obtained with dry mycelium was probably due to inhibitory substances present in the dry mycelium affecting the growth of Saccharomyces cerevisiae [35]. Phenols, furans, carboxylic acids and salts, are potential inhibitors of alcoholic fermentation and have a negative effect on yeast growth, cellular membrane function, and glycolysis [36]. The dry mycelium of Aspergillus niger generated in the production of citric acid, contains traces of carboxylic acid, which can cause inhibition in the production of ethanol.
The production of ethanol was not significantly different (P>0.05) at 24 h of fermentation in substrates MUDP and MGSF (Figure 7). The same behavior was observed in studies by Turhan et al. [37], using yeast and carob extracts as nitrogen sources, with a final ethanol concentration of 45 gL-1. Substrate MMAN showed statistically significant differences (P<0.05) at 24 h of fermentation, when compared with substrates MUDP and MGSF. The use of guava seed flour as the nitrogen source yielded 43.58% more ethanol than similar studies by Peña et al. [26]. On the other hand, Tang et al. [38] reported an ethanol concentration of 67 gL-1 at 48 h of fermentation using a substrate made of molasses and ammonium sulfate. Although guava seed flour contains 2% less protein than soy hulls, it yielded ethanol concentration 2.21 times higher than that of soy hulls fermented for 9 h [39].
The ethanol yield using MMAN substrate (36 gL-1) was similar to results by Peña et al. [26] using 250 gL-1 substrate supplemented with peptone (0.5 gL-1) and yeast extract (10 gL-1), and final ethanol concentration was 31 gL-1. These results indicate that the dry mycelium of Aspergillus niger can be a low cost alternative for ethanol production using recombinant strains of Saccharomyces cerevisiae.
The improvement of fermentation processes, efficient use of renewable resources, better reactor designs and selection of suitable substrates, are important to enhance growth and cell development [3], which will lead to a better use of raw materials increasing performance and productivity.
4. CONCLUSIONS
Guava seed flour is a good alternative nitrogen source in alcoholic fermentations. When MGSF was used as a substrate for fermentation, higher substrate conversions and better ethanol yield were observed. The use of this nitrogen source can reduce operating costs in ca. 35%, compared to current sources.
The lipids in guava seed flour showed antifoaming effects during the fermentation stage, and the vitamin and mineral content in guava seeds favored the growth and viability of the yeast, generating an added value to these agro-industrial wastes.
The MMAN substrate can be used in future research where the final concentration of ethanol needed is lower than 36 gL-1, or it can be pre-treated to eliminate traces of citric acid that inhibit fermentation.
Although guava seed flour was found to be a good alternative, the production of large volumes of sludge, due to the amount of flour needed to meet the nitrogen requirements, must be considered as a potential disadvantage.
ACKNOWLEDGMENTS
The authors acknowledge the International Center of Tropical Agriculture (CIAT) and the Sucromiles S.A. and Dulces San Antonio la Tentación companies for their support in this research.
REFERENCES
[1] Kwon, S., Lee, P.CH., Lee, E.G., Chang, Y.K. and Chang, N., Production of lactic acid by Lactobacillus rhamnosus with vitamin-supplemented soybean hydrolysate, Enzyme Microb Tech, 26, pp. 209-215, 2000. [ Links ]
[2] Serna, L. and Rodríguez, A., Economic production of lactic acid utilizing harvest wastes and sugar cane juice (Saccharum officinarum L.), Agr Tec, 67, pp. 29-38, 2007. [ Links ]
[3] Calderón, N., Evaluation of the use of antibiotics as a mechanism for the control of bacterial contaminants in fermentation for the production of ethyl alcohol [Undergraduate work]. Bogotá, DC: Javeriana Catholic University, 2007. [ Links ]
[4] Acevedo, A., Godoy, R. and Bolaños, G., Increase in the production of alcohol in molasses fermentation through the utilization of the enzymatic complex Rhyzozyme. XXII Colombian Congress of Chemical Engineering. Bucaramanga, Colombia, August 2003. [ Links ]
[5] Altaf, MD., Naveena, B.J. and Reddy, G., Screening of inexpensive nitrogen sources for production of L(+) lactic acid from starch by amylolytic Lactobacillus amylophilus GV6 in single step fermentation, Food Technol Biotech, 43(3), pp. 235-239, 2005. [ Links ]
[6] Bernardino-Nicanor, A., Añón, M.C., Scilingo, A.A. and Dávila-Ortíz, G., Functional properties of guava seed glutelins, J. Agri. Food Chem., 53(9), pp. 3613-3617, 2005. [ Links ]
[7] Bernardino-Nicanor, A., Scilingo, A., Añón, M.C. and Dávila-Ortíz, G., Guava seed storage protein: Fractionation and characterization, LWT Food Sci. Technol., 39, pp. 902-910, 2006. [ Links ]
[8] Chaplaa, D., Divechab, J., Madamwara, D. and Shaha, A., Utilization of agro-industrial waste for xylanase production by Aspergillus foetidus MTCC 4898 under solid state fermentation and its application in saccharification, Biochem. Eng. J., 49, pp. 361-369, 2010. [ Links ]
[9] Steinhaus, M., Sinuco, D., Polster, J., Osorio, C. and Schieberle, P., Characterization of the aroma-active compounds in pink guava (Psidium guajava, L.) by application of the aroma extract dilution analysis, J. Agric. Food. Chem., 56 (11), pp. 4120-4127, 2008. [ Links ]
[10] Elizalde, M.P. and Hernández, V., Guava seed as an adsorbent and as a precursor of carbon for the adsorption of acid dyes, Bioresour. Technol., 100, pp. 2111-2117, 2009. [ Links ]
[11] Nullvalue El tiempo.com.12000 Familias que viven de la guayaba en sur de Santander. Available: http://www.eltiempo.com/archivo/documento/MAM-553919 [mentioned 2 August 2011] [ Links ].
[12] Srivastava, S., Modi, D.R. and Garg, S.K., Production of ethanol from guava pulp by yeast strains, Bioresour. Technol., 60(3), pp. 263-265, 1997. [ Links ]
[13] Dhillona, G.S., Brara, S.K., Vermab, M. and Tyagi, R.D., Utilization of different agro-industrial wastes for sustainable bioproduction of citric acid by Aspergillus niger, Biochem. Eng. J., 54, pp. 83-92, 2011. [ Links ]
[14] Rabelo, S.C., Carrere, H., Filho, M. and Costa, A.C., Production of bioethanol, methane and heat from sugarcane bagassein a biorefinery concept, Bioresour. Technol., 102, pp. 7887-7895, 2011. [ Links ]
[15] Méndez, R., Guerrero, B., Jesús, R. and O'Callaghan, J., Estudio preliminar del Aspergillus niger como componente del pienso para ratas de laboratorio, Agroalimentación & Desarrollo Sustentable, 1(5), pp. 01-06, 2004. [ Links ]
[16] Cai, J., Yang, J., Du, Y., Fan, L., Qiu, Y., Li, J. and Kennedy, J., Enzymatic preparation of chitosan from the waste Aspergillus niger mycelium of citric acid production plant, Carbohydr. Polym., 64, pp. 151-157, 2006. [ Links ]
[17] A.O.A.C. Official Methods of Analysis of the Association of Official Analytical Chemists. 15th Ed., Arlington, Virginia, USA, 1990. [ Links ]
[18] Kjeldahl, J., Neue methode zur bestimmung der stickstoffs in organischen korpern, J. Anal. Chem., 22, pp. 366-382, 1883. [ Links ]
[19] Leterme, P. and Estrada, F., Análisis de alimentos y forrajes: Protocolos de laboratorio, Universidad Nacional de Colombia, Palmira, 2010. [ Links ]
[20] Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. and Smith, F., Colorimetric method for determination of sugar and related substances, Anal. Chem., 28, pp. 350-356, 1956. [ Links ]
[21] Millar, G.L., Use of dinitrosalicylic acid reagent for determination of reducing sugar, Anal. Chem., 31, pp. 426-429, 1959. [ Links ]
[22] Govindaswamy, S. and Vane, L., Kinetics of growth and ethanol production on different carbon substrates using genetically engineered xylose-fermenting yeast, Bioresource Technol., 98, pp. 677-685, 2007. [ Links ]
[23] Zhu, S., Wu, Y., Yu, Z., Zhang, X., Wang, C., Yu, F. and Jin, S., Production of ethanol from microwave-assisted alkali pretreated wheat straw, Process Biochem., 41, pp. 869-873, 2006. [ Links ]
[24] Pleassas, S., Bekatorou, A., Koutinas, A., Soupioni, M., Banat, I. and Marchant, R., Use of Saccharomyces cerevisiae cells immobilized on orange peel as biocatalyst for alcoholic fermentation, Bioresource Technol., 98, pp. 860 -865, 2007. [ Links ]
[25] Monsalve, J.F., Medina, V.I. and Ruiz, A.A., Ethanol production of banana shell and cassava starch, DYNA, 150, pp. 21-27, 2006. [ Links ]
[26] Peña, C. and Arango, R., Evaluation of ethanol production using recombinant strains of Saccharomyces cerevisiae from sugar cane molasses, DYNA, 159, pp. 153-161, 2009. [ Links ]
[27] Rgyracz, G., Koczo, K. and Wasan, D., Mechanisms of antifoam deactivation, J. Colloid Interface Sci., 181, pp. 124-135, 1996. [ Links ]
[28] Vasco, N. L., Toro, J.F. and Padilla, S., Composición Química de la Semilla de Guayaba. II Encuentro: Participación de la mujer en la ciencia. Guanajuato, México, Mayo 2005. [ Links ]
[29] Echegaray, O.F., Carvalho, J.C.M., Fernandes, A.N.R., Sato, S., Aquarone, E. and Vitolo, M., Fed-batch culture of Sacchoromyces cerevisiae in sugar-cane blackstrap molasses: invertase activity of intact cells in ethanol fermentation, Biomass Bioenergy, 19, pp. 39-50, 2000. [ Links ]
[30] Kazuhiko, T. and Kozo, O., Factors affecting the ethanol productivity of yeast in molasses, J. Ferment. Bioeng., 9(5), pp. 449-452, 1995. [ Links ]
[31] Madigan, M.T. and Martinko, J.M., Dunlap, P.V. and Clark, D.P. Brock Biology of the Microorganisms, Pearson Education N.S.A., San Francisco, 2009. [ Links ]
[32] Yegres, F., Fernández-Zeppenfeldt, G., Padin, C., Rovero, L. and Richard-Yegres, R., Saccharomyces cerevisiae en la fabricación del licor cocuy, Rev. Soc. Ven. Microbiol., 23(1), 2003. [ Links ]
[33] El, D.M. and Yassen, A.A., Evaluation of utilization of guava seed meal (Psidium guajava L.) in cookies preparation as wheat flour substitute, Nahrung, 41(6), pp. 344-348, 1997. [ Links ]
[34] FAO Food and Agriculture Organization of the United Nations. Available: http://fao.org/inpho/content/documents/vlibrary/AE620s [mentioned August 1st, 2011] [ Links ].
[35] Palmqvist, E., Hahn-HiigerdaL, B., Galbe, M. and Zacchi, G., The effect of water-soluble inhibitorsfrom steam-pretreated willow on enzymatic hydrolysis and ethanol fermentation, Enzyme Microb. Technol., 19, pp. 470-476, 1996. [ Links ]
[36] Klinke, H., Thomsen, A.B. and Ahring, B.K., Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass, Appl. Microbiol. Biotechnol., 66, pp. 10-26, 2004. [ Links ]
[37] Turhan, I., Bialka, K.L., DemircI, A. and Karhan, M., Ethanol production from carob extract by using Saccharomyces cerevisiae., Bioresour. Technol., 101, pp. 5290-5296, 2010. [ Links ]
[38] Tang, Y., An, M., Zhong, Y.L., Shigeru, M., Wu, X. and Kida, K., Continuous ethanol fermentation from non-sulfuric acid-washed molasses using traditional stirred tank reactors and the flocculating yeast strain KF-7, J. Biosci. Bioeng., 109(1), pp. 41-46, 2010. [ Links ]
[39] Mielenz, J.R., Bardsley, J.S. and Wymana, C.E., Fermentation of soybean hulls to ethanol while preserving protein value, Bioresour. Technol., 100, pp. 3532-3539, 2009. [ Links ]