Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.80 no.180 Medellín July/Aug. 2013
LAMINAR BURNING VELOCITY OF NATURAL GAS/SYNGAS-AIR MIXTURE
VELOCIDAD DE DEFLAGRACIÓN LAMINAR DE LA MEZCLA GAS NATURAL/GAS DE SÍNTESIS CON AIRE NORMAL
CÉSAR CARDONA
University of Antioquia, Master of Engineering student, ccardona@udea.edu.co
ANDRÉS AMELL
University of Antioquia, GASURE group Coordinator, anamell@udea.edu.co
HUGO BURBANO
University of Antioquia, Associate Researcher, hugoburbano@udea.edu.co
Recibido para revisar December 11th , 2011, aceptado March 15th, 2013, versión final March 21th, 2013.
ABSTRACT: This study suggests the equimolar mixture of Natural Gas (100% CH4) and Synthesis Gas (40% H2 + 40% CO + 20% CO2) as an alternative to reduce hydrocarbons consumption and reduce pollutant emissions. As a key parameter to characterize this combustible mixture, the laminar burning velocity was studied based on numerical simulations and experimental measurements in flames generated using a contoured slot-type nozzle burner and the Schlieren technique, varying the air-fuel ratio at standard temperature and pressure. It was found that the flame speed of the equimolar mixture increases with respect to that of pure methane. This behavior can be explained by the presence of hydrogen in the fuel mixture, which has a direct effect on the combustion kinetics, generating H and OH radicals that increase the global reaction rate of the mixture and consequently the burning velocity.
KEYWORDS: Laminar burning velocity, Atmospheric burner, Natural Gas, Synthesis Gas.
RESUMEN: En este estudio se propone la mezcla equimolar de gas natural (100%CH4) y gas de síntesis (40%H2 + 40%CO + 20%CO2) como alternativa para reducir el consumo de hidrocarburos y reducir las emisiones contaminantes. Como parámetro principal para caracterizar esta mezcla combustible, se estudió la velocidad de deflagración laminar a partir de simulaciones de cinética detallada y mediciones experimentales en llamas generadas usando un quemador de perfil contorneado e imágenes Schlieren obtenidas a condiciones normales de presión y temperatura, variando la relación aire-combustible. Se encontró que la velocidad de deflagración de la mezcla equimolar aumenta con respecto a la del metano puro y puede ser explicado a través de la presencia de hidrógeno en la mezcla combustible, que tiene un efecto directo en la cinética de la combustión, generando radicales H y OH que aumentan las tasas de reactividad de la mezcla y consecuentemente la velocidad de quemado del combustible.
PALABRAS CLAVE: Velocidad de deflagración laminar, Quemador atmosférico, Gas Natural, Gas de Síntesis.
1. INTRODUCTION
Currently, the proved reserves of world’s natural gas are considerable and represent 25% of global primary energy consumption [1], but its non-renewable nature is motivating the search for better usage methods, from the design of more efficient equipment to the mixing of this hydrocarbon with renewable or alternative fuels, in order to improve its combustion properties or to use the resources at a lower rate.
In Colombia, natural gas demand is expected to increase at an annual rate of 4.0% for the period 2011-2020, but the country can be self-sufficient until 2019 [2]. This trend, coupled with the global concern of pollution effects due to the use of fossil fuels, promotes the combined use of these fuels with others of renewable nature. In this paper, the feasibility of using mixtures of methane (natural gas) and hydrogen/carbon monoxide (synthesis gas) is studied. Synthesis gas or "syngas" is produced from gasification of solid fuels such as coal, biomass, organic waste and refinery waste. In Colombia, syngas is expected to play an important role in the diversification of the energy supply, since coal reserves are the largest in Latin America, not to mention the considerable amount of biomass for gasification.
Synthesis gas is mainly composed of hydrogen (H2) and carbon monoxide (CO) in addition to carbon dioxide (CO2), nitrogen (N2), water vapor (H2O), methane (CH4) and other hydrocarbons [3]; the amount of each of these compounds in the mixture varies depending on the gasification method and the raw material used [4]. The importance of this gas lies in the wide availability of suitable raw materials for its production, in addition to the low pollution levels resulting from its combustion. But the biggest concern related to its use, is the variation in composition [5]. Natural gas, meanwhile, is composed basically of methane (CH4) and small amounts of ethane (C2H6), propane (C3H8), butane (C4H10), among others [6].
The characteristics of the combustion of natural gas and syngas have been widely studied as individual components. For this reason, investigations should be performed to predict the behavior of their combustion as a mixture, in order to establish the combustion properties that are to be used, for example, for thermal equipment design. One of the most important combustion properties is the laminar burning velocity (SL),, defined as the speed with which the unburnt gases move towards the flame front [7; 8]. This parameter, characteristic of each fuel mixture, contains essential information related to its reactivity and diffusivity; it is also used for the analysis of combustion phenomena such as the stability and structure of premixed flames, flashback, blow off and extinction, turbulent burning velocity and the validation of reaction mechanisms [5].
The idea of mixing fuels with others to improve their combustion properties is not new: hydrogen [9; 10; 11; 8; 12; 13; 14], propane [15] and carbon monoxide [16; 17], have been mixed with methane. The variation of pressure and temperature of the unburnt gases [18; 19; 20] and the percentage of CO, CO2, N2 in the mixture [21; 22; 23; 24; 25; 26; 27] have been shown to have strong influence on syngas burning velocity.
The combustion properties of syngas and methane have been reported in few publications: in 1959, Scholte and Vaags [16] reported the laminar burning velocity for six different mixtures of H2/CO/CH4; Wierzba [28] found SL values for three different mixtures by varying the unburnt gases temperature up to 300°C; Cong and Dagout [29] studied the oxidation of CH4/CO/H2 mixtures diluted in nitrogen in a jet-stirred reactor; Manh [30] reported the instabilities of a 50H2/50CO mixture while adding different percentages of hydrocarbons, including methane; Alavandi [31] studied five mixtures with equal amounts of H2 and CO (by volume) while the methane percentage varied from 100% to 0% in a porous burner; it was found that the addition of syngas to methane significantly reduces the NOx and CO production; Saxena [32] reported the influence of the amount of H2 and CO on the combustion of methane. None of the studies mentioned above included the combustion of methane with a real synthesis gas mixture, i.e., containing N2 or CO2 in its composition. In practical cases, the presence of inerts in the fuel should be taken into account.
The main objective of the this investigation is to determine numerically and experimentally the laminar burning velocity of the equimolar mixture of natural gas (100% CH4) and synthesis gas (40% H2 + 40% CO + 20% CO2) at lean and rich equivalence.
Experiments were conducted with the burner stabilized flame technique and values of SL were determined with the angle method through Schlieren images of the flame front.
2. EXPERIMENTAL METHODOLOGY
2.1. Experimental Setup
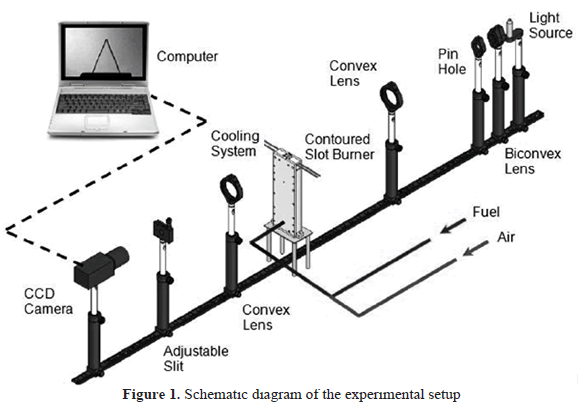
Figure 1 shows a schematic image of the experimental setup. The flames were generated in a burner with a contoured slot-type nozzle (21 mm x 7.2 mm). This burner allows laminar flows to be maintained for all the equivalence ratios studied and also helps to reduce the effects of flame stretch and curvature on the axis of the burner [27]. Additionally, a cooling circuit was implemented inside the burner in order to keep the unburnt gases at constant temperature.
To accurately reproduce the air-fuel mixtures, high purity certified gases were used. The corresponding flow rates for each of the components of the mixture were measured in specially calibrated rotameters. A uniform mixing of the gases was guaranteed before they reached the burner inlet.
To obtain the Schlieren images, a high-intensity Xenon lamp was used as a light source. A biconvex lens (diameter 50.8 mm, 38.1 mm focus length) and a pin hole were used to focus the light that was subsequently sent out to the test zone using a planar-convex lens (diameter 50.8 mm, focus length 250 mm). Finally, another planar-convex lens was used to focus the deflected and undeflected rays. An adjustable slit blocked the deflected rays while the undeflected rays were captured with a high resolution CCD Camera (Basler scA1400-30 gm, 1392 x 1040 pixels, 30 fps). The high-resolution images were obtained using a Macro Camera lens (Sigma, diameter 72 mm, focus length 150 mm, f/2.8) and transmitted to a computer to be monitored and eventually captured.
2.2. Determination of the Laminar Burning Velocity
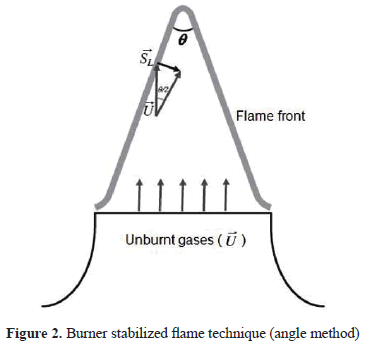
To determine the laminar burning velocity the angle method was used. The measurement is based on the principle that the velocity at the nozzle exit of the unburnt gases is equal to the velocity at which the flame front propagates from the burnt to the unburnt zone at an angle q as shown in Figure 2. The laminar burning velocity is related to q according to (1):

where U is the mean velocity of the unburnt gases at the exit of the burner. Although in this method it is only possible to determine average values of due to the effect of the flame stretch and curvature and heat loss to the walls of the burner, it has been demonstrated that a well-designed contoured slot-type nozzle burner can yield highly accurate laminar burning velocity values, as demonstrated by Burbano et al. [27] and Pareja et al. [33; 27; 34].
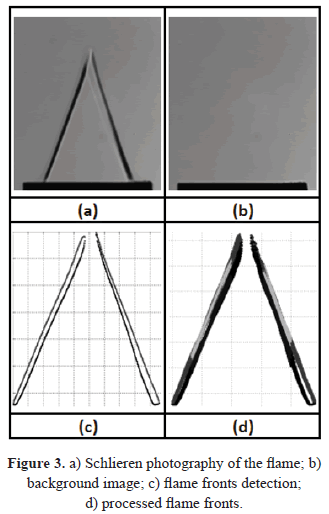
For each flame it is possible to determine two q angles, corresponding to the inner and outer edges of the flame front, which are obtained through the processing of high resolution images. The processing consist in subtracting from the flame image (Figure 3a) a background image previously captured (Figure 3b), the contrast of the resulting image was increased in order to detect the flame fronts (Figure 3c). It was found that the difference between these two angles is less than 1° and therefore, the values reported here correspond to the inner flame front angle.
2.3. Experimental Conditions
The experiments were performed in the city of Medellín Colombia at 0.828 atm, 68% relative humidity and 295 ± 2 K. The equivalence ratios varied from lean ( f = 0.8) to rich conditions ( f = 1.4). Within this range, it was possible to obtain well-defined and stable flames. In order to avoid undesirable phenomena, such as flashback and blow off, an adequate unburnt gas velocity (U), must be chosen. This velocity is 2 to 4 times the calculated laminar burning velocity as explained in the next section.
The measured variables were the mean velocity of the unburnt gases and the angle of the flame. For each equivalence ratio, 50 images were captured and processed in order to obtain reliable data. Figure 3d shows the resulting image after processing all the flame fronts. Errors in the measurement of the laminar burning velocity for each equivalence ratio were calculated from the propagation error of the mean velocity measurements of the unburnt gases at the exit of the burner and the flame angle.
The average estimated error of the mean velocity is 1.45 cm/s which was calculated from the measurement errors of the burner nozzle area and the flow of fuel and air. The error of the flame angle was calculated from the statistical treatment of the 50 images captured for each case, where standard deviations were less than 1.5°.
3. NUMERICAL METHODOLOGY
The numerical calculations were performed using the one-dimensional premixed flame code PREMIX of the CHEMKIN-PRO package. For comparative purposes, simulations were carried out with two detailed kinetic mechanisms, GRI-Mech 3.0 [35], with effectiveness for the oxidation of methane verified in several studies [11; 36; 37; 35] and USC-Mech II, developed for the combustion of H2/CO/C1-C4 compounds [22] and the result of updating the H2/CO combustion model proposed by Mueller et al. [38]. The transport properties were evaluated using the model of multicomponent diffusion and due to the presence of hydrogen in the studied mixture, thermal diffusion was also considered (Soret effect). As has been reported, the accuracy of the calculated SL depends on the spatial resolution of the simulations [39; 33]; when using a low number of grid points, an error of 5 to 10% can be expected. For this reason, according to Dlugogorski et al. [40], the GRAD and CURV values were set lower than 0.01 to generate a grid of more than 1000 points. Thus SL values converged and the flame temperature approached the adiabatic flame temperature.
4. RESULTS AND DISCUSSION
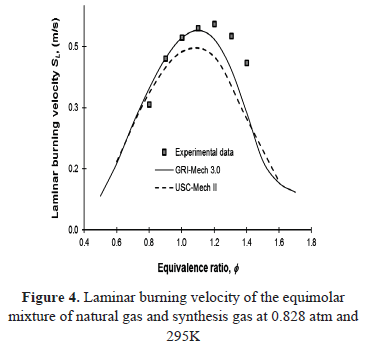
The experimental variation of the laminar burning velocity of the equimolar mixture of natural gas and synthesis with equivalence ratio is shown in Figure 4, along with the results of the numerical simulations carried out using the detailed kinetic mechanisms GRI-Mech 3.0 and USC-Mech II. The volumetric composition of the mixture corresponds to 50% CH4, 20% H2, 20% CO and 10% CO2.
The laminar burning velocity values are higher than those predicted by the mechanisms, however, with lean conditions, experimental results agree relatively well with the results obtained from GRI-Mech 3.0. Under rich conditions and for the region where maximum laminar burning velocities were attained, both mechanisms failed to reproduce the experimental values. Note that the USC-Mech II mechanism does not fit any region of the experimental data and only shows good agreement in the lean region compared to the results of GRI-Mech 3.0.
The differences between numerical and experimental results can be explained as the mechanisms have been adjusted for the oxidation of methane and hydrogen/carbon monoxide separately, and to the best of the authors’ knowledge, the mixture of the two has never been used to adjust any of the mechanisms used here.
This behavior had already been registered by Natarajan et al. [23], where the same mechanisms were subjected to testing with different mixtures of H2/CO with inert dilution at different rates. It was evidenced that GRI-Mech 3.0 tends to fit better to the experimental data.
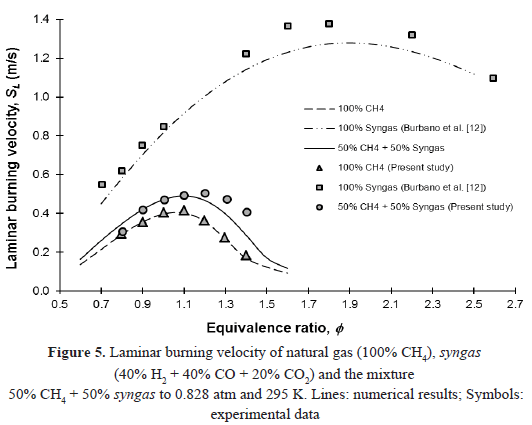
The laminar burning velocity of the equimolar mixture of natural gas and syngas is higher than the one of natural gas (pure methane), as shown in Figure 5, where the experimental data, along with the results of simulation with Gri-Mech 3.0 are presented. This behavior can be explained because of the effect of H2/CO addition to the mixture. Halter [11], Cong and Dagout [29] and Saxena [32] have shown that the presence of hydrogen in the mixture results in an increase in the burning velocity and this is not due to changes in flame temperature, as the adiabatic flame temperature only varies slightly with the addition of hydrogen. For comparison purposes, the results obtained by Burbano et al. [33] for the syngas mixture (40% H2 + 40% CO + 20% CO2), a chemical composition corresponding approximately to that obtained by E-gas Conoco-Phillips coal gasification technology [41], are also presented. These results were obtained under the same experimental conditions discussed in previous sections.
Glassman [42] reported a simple model of the laminar flame speed to explain this behavior which is presented in equation (2).

where a is the thermal diffusivity, RR is the global reaction rate and r is the density of the unburnt gases. When adding syngas to methane the thermal diffusivity is kept constant (a variation of 0.16%), the overall reactivity of the mixture increases because of the high hydrogen content and the reactant density decreases by 1.22% due to its low molecular weight; this behavior results in higher burning velocities.
The presence of CO and H2 in the fuel mixture promotes the reaction CO + OH = H + CO2 producing H radicals; consequently, the production of OH radicals through H + O2 = OH + H increases. The net increase in the concentration of OH and H in the presence of CO and H2 increases methane consumption rate, in other words, the addition of H2/CO to CH4 increases methane reactivity.
With the increase of SL, for the natural gas/syngas mixture, the blow off tendency is expected to improve when compared to a flame of pure methane. However, further analysis of the inherent instabilities must be carried out in order to understand the behavior of the CH4/H2/CO flames.
5. CONCLUSIONS
Measurements of the laminar burning velocity of the equimolar mixture of natural gas (100% CH4) and synthesis gas (40% H2 + 40% CO + 20% CO2) were made at 0.828 atm, room temperature and different equivalence ratios, varying from f = 0.8 to f = 1.4. Numerical calculations of the laminar burning velocity were also performed using detailed reaction mechanisms and were compared with experimental results. Numerical calculations and experimental measurements for natural gas and synthesis gas were also presented for comparative purposes. From the analysis of the results, the following conclusions can be stated:
The increase in the laminar burning velocity of the equimolar mixture, compared to that of the pure methane, can be explained by the addition of H2/CO to the mixture. Several authors (Halter [11], Cong and Dagout [29] and Saxena [32]) agree that the presence of hydrogen in the fuel promotes the formation of OH radicals through H2/O2 system. If the amount of H2 in the mixture increases, the concentrations of H and OH grows, increasing the reactivity of the mixture and therefore, the laminar burning velocity. In this case, the maximum value of the laminar burning velocity experimentally determined for the equimolar mixture (50.45 cm/s at f = 1.2) was found to be higher than that of pure methane (41.65 cm/s at f = 1.1) but lower to that of pure syngas (137.68 cm/s at f = 1.8).
At stoichiometric and lean conditions, the numerical results are very well adjusted to experimental data, within 4% and 8% deviation for GRI-Mech 3.0 and USC-Mech II respectively. However, for the equimolar mixture at rich conditions, the variations are 30% and 33% for GRI-Mech 3.0 and USC-Mech II. The data suggest that the current mechanisms should be re-examined for rich conditions in CH4/H2/CO mixtures.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the GASURE group and the program "Sostenibilidad 2011-2012" of the University of Antioquia for the valuable economic contribution for the development of this research. The support of COLCIENCIAS through the financing of the project "Laminar Burning Velocities of Natural Gas/Synthesis Gas mixtures: Numerical and Experimental Study " is gratefully acknowledged too. The authors also thank the "Jóvenes Investigadores U. de A." for their support during the experimental measurements.
REFERENCES
[1] CIA. The world factbook. Available:
[2] UPME. Informe de Rendición de Cuentas 2010, Bogotá. Unidad de Planeación Minero Energética, 2010. [ Links ]
[3] Casleton, K. H., R. W. Breault and G. A. Richards. System Issues and Tradeoffs Associated with Syngas Production and Combustion, Combust Sci Technol, 180(6), 1013-1052, 2008. [ Links ]
[4] Lieuwen, T. C., V. Yang and R. Yetter. Synthesis Gas Combustion: Fundamentals and Applications, CRC Press, Boca Raton, 2010. [ Links ]
[5] Monteiro, E., M. Bellenoue, J. Sotton, et al. Laminar Burning Velocities and Markstein Numbers of Syngas-Air Mixtures, Fuel, 89(8), 1985 - 1991, 2010. [ Links ]
[6] NGSA. Natural Gas. Available: http://www.naturalgas.org/overview/background.asp. [Citado: Agosto 2011] [ Links ].
[7] Amell, A., J. García, A. Quilindo, et al. Influencia de la Altitud Sobre la Velocidad de Deflagración del Gas Natural, Rev Fac Ing, 32, 72-81, 2004. [ Links ]
[8] Huang, Z., Y. Zhang, K. Zeng, et al. Measurements of Laminar Burning Velocities for Natural Gas-Hydrogen-Air Mixtures, Combust Flame, 146(1-2), 302-311, 2006. [ Links ]
[9] Milton, B. E. and J. C. Keck. Laminar Burning Velocities in Stoichiometric Hydrogen and Hydrogen-Hydrocarbon Gas Mixtures, Combust Flame, 58(1), 13-22, 1984. [ Links ]
[10] Karim, G. A., I. Wierzba and Y. Al-Alousi. Methane-Hydrogen Mixtures as Fuels, Int J Hydrogen Energy, 21(7), 625-631, 1996. [ Links ]
[11] Halter, F., C. Chauveau, N. Djebaïli-Chaumeix, et al. Characterization of the Effects of Pressure and Hydrogen Concentration on Laminar Burning Velocities of Methane-Hydrogen-Air Mixtures, Proc Combust Inst, 30(1), 201-208, 2005. [ Links ]
[12] Ilbas, M., A. P. Crayford, I. Yilmaz, et al. Laminar Burning Velocities of Hydrogen-Air and Hydrogen-Methane-Air Mixtures: An Experimental Study, Int J Hydrogen Energy, 31(12), 1768-1779, 2006. [ Links ]
[13] Bunev, V., V. Babkin, A. Baklanov, et al. Selective Oxidation of Hydrogen in Rich Hydrogen-Methane-Air Flames, Combust Explos Shock Waves, 43(5), 493-500, 2007. [ Links ]
[14] Burbano, H., A. Amell and J. García. Effects of Hydrogen Addition to Methane on the Flame Structure and CO Emissions in Atmospheric Burners, Int J Hydrogen Energy, 33, 3410-3415, 2008. [ Links ]
[15] Petersen, E. L., D. M. Kalitan, S. Simmons, et al. Methane/Propane Oxidation at High Pressures: Experimental and Detailed Chemical Kinetic Modeling, Proc Combust Inst, 31, 447-454, 2007. [ Links ]
[16] Scholte, T. and P. Vaags. Burning Velocities of Mixtures of Hydrogen, Carbon Monoxide and Methane with Air, Combustion and Flame, 3(0), 511-524, 1959. [ Links ]
[17] El-Sherif, S. A. Control of Emissions by Gaseous Additives in Methane-Air and Carbon Monoxide-Air Flames, Fuel, 79(5), 567-575, 2000. [ Links ]
[18] Bunkute, B. and J. B. Moss. Laminar Burning Velocities of Carbon Monoxide/Hydrogen - Air Mixtures at High Temperatures and Pressures, 3rd European Combustion Meeting, 2007. [ Links ]
[19] Cavaliere, D. E., M. D. Ioannon, P. Sabia, et al. A Comprehensive Kinetic Modeling of Ignition of Syngas/Air Mixtures at Low Temperatures and High Pressures. 32nd Meeting on Combustion, Italy, 2009. [ Links ]
[20] Natarajan, J., Y. Kochar, T. Lieuwen, et al. Pressure and Preheat Dependence of Laminar Flame Speeds of H2/CO/CO2/O2/He Mixtures, Proc Combust Inst, 32, 1261-1268, 2009. [ Links ]
[21] McLean, I. C., D. B. Smith and S. C. Taylor. The Use of Carbon Monoxide/Hydrogen Burning Velocities to Examine the Rate of the CO+OH Reaction, Symposium (International) on Combustion, 25(1), 749-757, 1994. [ Links ]
[22] Davis, S. G., A. V. Joshi, H. Wang, et al. An Optimized Kinetic Model of H2-CO Combustion, Proc Combust Inst, 30, 1283-1292, 2005. [ Links ]
[23] Natarajan, J., S. Nandula, T. Lieuwen, et al. Laminar Flame Speeds Of Synthetic Gas Fuel Mixtures ASME Turbo Expo 2005: Power for Land, Sea and Air Reno-Tahoe, Nevada, USA 2005. [ Links ]
[24] Frassoldati, A., T. Faravelli and E. Ranzi. The Ignition, Combustion and Flame Structure of Carbon Monoxide/Hydrogen Mixtures. Note 1: Detailed Kinetic Modeling of Syngas Combustion Also in Presence of Nitrogen Compounds, Int J Hydrogen Energy, 32(15), 3471-3485, 2007. [ Links ]
[25] Natarajan, J. Laminar Flame Speeds of H2/CO Mixtures: Effect of CO2 Dilution, Preheat Temperature and Pressure, Combustion and Flame, 151, 104-119, 2007. [ Links ]
[26] Natarajan, J., T. Lieuwen and J. Seitzman. Laminar Flame Speeds and Strain Sensitivities of Mixtures of H2 with CO, CO2 and N2 at Elevated Temperatures. Turbo Expo 2007: Power for Land, Sea and Air, Montreal, Canada, 2007. [ Links ]
[27] Burbano, H. J., J. Pareja and A. A. Amell. Laminar Burning Velocities and Flame Stability Analysis of H2/CO/Air Mixtures with Dilution of N2 and CO2, Int J Hydrogen Energy, 36(4), 3232-3242, 2011. [ Links ]
[28] Wierzba, I. and Q. Wang. The Flammability Limits of H2-CO-CH4 Mixtures in Air at Elevated Temperatures, Int J Hydrogen Energy, 31(4), 485-489, 2006. [ Links ]
[29] Le Cong, T. and P. Dagaut. Experimental and Detailed Kinetic Modeling of the Oxidation of natural Gas, Natural Gas/Syngas Mixtures and Effect of Burnt Gas. Third European Combustion Meeting. 1-6, 2007. [ Links ]
[30] Manh, T., J. Park, O. Kwon, et al. Effects of hydrocarbon addition on cellular instabilities in expanding syngas-air spherical premixed flames, International Journal of Hydrogen Energy, 34, 6961 - 6969, 2009. [ Links ]
[31] Alavandi, S. K. and A. K. Agrawal. Experimental Study of Combustion of Hydrogen-Syngas/Methane Fuel Mixtures in a Porous Burner, Int J Hydrogen Energy, 33(4), 1407-1415, 2008. [ Links ]
[32] Saxena, P. and K. Seshadri. The Influence of Hydrogen and Carbon Monoxide on Structure and Burning Velocity of Methane Flames. Fall Technical Meeting of the Western States Section of the Combustion Institute, Irvine, CA, 2009. [ Links ]
[33] Burbano, H. J., J. Pareja and A. A. Amell. Laminar Burning Velocities and Flame Stability Analysis of Syngas Mixtures at Sub-Atmospheric Pressures, Int J Hydrogen Energy, 36(4), 3243-3252, 2010. [ Links ]
[34] Pareja, J., H. J. Burbano, A. Amell, et al. Laminar Burning Velocities and Flame Stability Analysis of Hydrogen/Air Premixed Flames at Low Pressure, Int J Hydrogen Energy, 36(10), 6317-6324, 2011. [ Links ]
[35] Smith, G. P., D. M. Golden, M. Frenklach, et al. GRI-Mech 3.0. Available: http://www.me.berkeley.edu/gri_mech/. [Citado:8 abril 2013] [ Links ].
[36] Powell, O. A., P. Papas and C. Dreyer. Laminar Burning Velocities for Hydrogen-, Methane-, Acetylene-, and Propane-Nitrous Oxide Flames, Combust Sci Technol, 181(7), 917 - 936, 2009. [ Links ]
[37] Selle, L., T. Poinsot and B. Ferret. Experimental and Numerical Study of the Accuracy of Flame-Speed Measurements for Methane/Air Combustion in a Slot Burner, Combust Flame, 158(1), 146-154, 2010. [ Links ]
[38] Mueller, M. A., R. A. Yetter and F. L. Dryer. Flow Reactor Studies and Kinetic Modeling of the H2/O2/NOX and CO/H2O/O2/NOX Reactions, International Journal of Chemical Kinetics, 31(10), 705-724, 1999. [ Links ]
[39] Dong, Y., C. M. Vagelopoulos, G. R. Spedding, et al. Measurement of laminar flame speeds through digital particle image velocimetry: Mixtures of methane and ethane with hydrogen, oxygen, nitrogen, and helium, Proc Combust Inst, 29(2), 1419-1426, 2002. [ Links ]
[40] Dlugogorski, B. Z., R. K. Hichens, E. M. Kennedy, et al. Propagation of Laminar Flames in Wet Premixed Natural Gas-Air Mixtures, Process Safety and Environmental Protection, 76(2), 81-89, 1998. [ Links ]
[41] Chen, L.-M., V. Dhar, J. Guo, et al. Design Toward Integrat ion of CO2 Capture and Fuel Conversion Technologies for a 500 MWe Coal-Based Power Plant, EGEE 580. CO2 Capture Group, 2006. [ Links ]
[42] Glassman, I., Combustion, Academic Press, New York, 1996. [ Links ]