Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.183 Medellín jan./fev. 2014
https://doi.org/10.15446/dyna.v81n183.34802
http://dx.doi.org/10.15446/dyna.v81n183.34802
MICROWAVE HEATING AND SEPARATION OF WATER-IN-OIL EMULSION FROM MEXICAN CRUDE OIL
CALENTAMIENTO POR MICROONDAS Y LA SEPARACIÓN DE EMULSIÓN DE AGUA EN ACEITE DEL PETRÓLEO CRUDO MEXICANO
ADRIAN VAZQUEZ V.
Dr(Est.)Inst Politécnico Nacional, México, Centro de Investigación Cienc. avazquezv0802@alumno.ipn.mx
ARTURO LOPEZ M.
Profesor Titular Inst Politécnico Nacional, México, Centro de Inve Cienc. Avanzada, arlopezm@ipn.mx
LUIS J. ANDRADE C.
MSc(Est) Inst Politécnico Nacional, México, Centro de Inv. en Ciec. landradec1100@alumno.ipn.mx
ARIANA M. VAZQUEZ A.
MSc(Est) Inst Politécnico Nacional, México, Centro de Inv. en Ciencia , ariana_vazquez@hotmail.com
Received for review February 04th, 2013, accepted October 8th, 2013, final version December, 04th, 2013
ABSTRACT: Microwave heating and gravity sedimentation are alternatives for demulsification and layer separation into oil and water layers, this process was demonstrated in the laboratory and provides an option for reducing and oil recovering from water-in-oil Mexican oil emulsions.
The combinatorial process was implemented in a test lab using Mexican crude oil samples. The Laboratory samples were 100% and 50-50%, crude and crude-water respectively, were heated. The results were encouraging show that microwave heating and gravity sedimentation are alternatives for the separation of Mexican Oil emulsions.
Keyword: Microwave heating, Demulsification, Separation, Water-in-oil emulsions.
RESUMEN: El calentamiento por microondas y la sedimentación por gravedad son alternativas para desemulsificar y separar por capas aceite y agua, este proceso se demostró en el laboratorio y proporciona una opción para la reducción y la recuperación de emulsiones de agua en aceite del petróleo Mexicano.
El proceso se implementó en un laboratorio de pruebas con muestras de petróleo crudo mexicano. Las muestras de laboratorio fueron del 100% y 50-50%, crudo y crudo-agua, respectivamente, se calentaron. Los resultados fueron alentadores muestran que el calentamiento por microondas y la sedimentación por gravedad son alternativas para la separación de emulsiones de petróleo mexicano.
Palabras Clave: Calentamiento por Microonda, Desemulsificar, Emulsión de agua en aceite.
1. INTRODUCTION
A significant portion of world crude oil is produced in the form of a water-in-oil emulsion stabilized by natural surfactants that must be treated (demulsified) before they can be processed [1].
Water and oil sometimes combine (emulsify) during industrial processes. Resulting emulsions are either oil-in-water (o/w) or water-in-oil (w/o), depending on which material is dispersed in the other. An oil-in-water emulsion has water as the continuous phase, while the water-in-oil emulsion has oil in the continuous phase. Either type of emulsion may contain other contaminating materials (solids, dirt, metal particles, emulsifiers, cleaners, soaps, solvents, etc.). Emulsions can be found in a variety of industries and formed by a variety of processes. Since formation of emulsions is so specific to the industry and process, the emulsion breaking product selection is somewhat difficult and requires bench testing [2]. For water-in-oil emulsions which usually have high viscosity, the required mixing of these chemicals with the emulsion is difficult. Also when a high dosage of chemicals is used to overcome the difficulty, it leads to a secondary pollutant, since the separated water may contain too high a level of chemicals to be discharged to public water.
The concept of microwave heating of emulsion was first introduced by Klaila (1978) [3] and Wolf (1986) [4] in their patent applications. Recently, research continues to develop technology for microwave demulsification chemical plants, as described by Coutinho (2010) [5].
Today the Mexican oil fields have a high content of water and oil, and the water usually contains dissolved salts forming brines.
The use of microwaves is an alternative, effective, clean and chemicals free method for oil desalting and dehydration. This publication shows that microwaves are an efficient alternative to heating oil emulsions.
2. EXPERIMENTAL
The study area is located in the oil drilling fields in Altamira, Tamaulipas, Mexico. Two types of sample of crude, 100% crude and 50-50% crude-water, were heated.
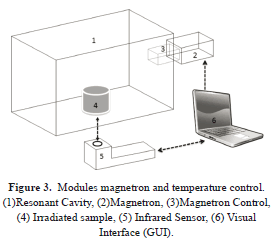
The variables studied are the temperature and the irradiation time during microwave irradiation. Equipment was built to irradiate emulsions and measure the temperature at a predetermined time. Modules were designed for measuring the temperature and controlling the magnetron.
The experimental process is: 1) Collection of Oil Field samples (Figure 4,7), 2) Preparation and Stabilization of emulsions of 100% and 50% crude and crude-water respectively, 3) 50 ml of both samples are irradiated.
Temperature Measurement Module
An infrared sensor (Raytek RAYTXSLTCF1 model) (Figure 1) for measuring the temperature of oil samples, together with a graphical interface. Which can measure temperature in real time while the samples are being irradiated.
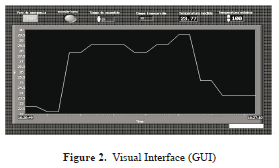
A graphic user interface (GUI) was designed to visualize the temperature inside the sample to be irradiated with microwaves in real time (see Figure 2).
Magnetron Control Module
The magnetron operates at a working frequency of 2.45 GHz and a rated power output of 700 W. The control module magnetron has a graphical interface which controls the magnetron, the duration of microwave irradiation and emergency protections (see Figure 2,3).
3. RESULTS AND DISCUSSION
1) Collection of Oil Field samples
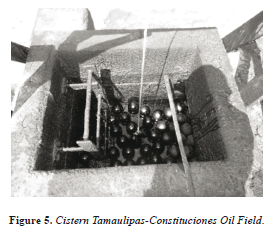
Samples were collected from a cistern in the Tamaulipas- Constituciones Oil Field (Figures 4 and 5).
2) Preparation and Stabilization of emulsions.
Two types of samples were prepared and stabilized, 50-50% crude-water (Figure 6) and 100% crude (Figure 7). Each sample contained 50 ml.
3) Irradiation of samples.
Oil-Water samples irradiated for 30 to 60 seconds
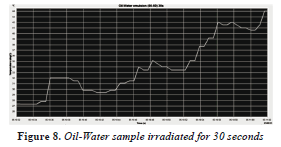
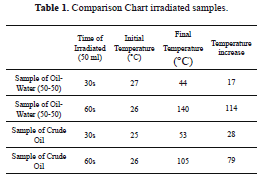
The sample of oil-water 50-50% was irradiated for 30 seconds (Figure 8), with an initial temperature of 27 °C and a final temperature of 44 °C, with a temperature increase of 17 °C, shown in the Table 1, there is a rapid increase in temperature.
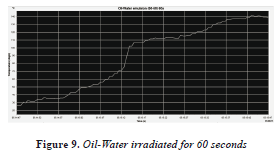
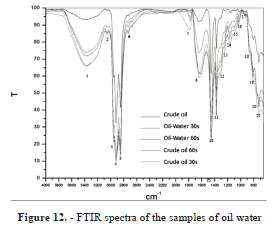
Then, the other sample of oil-water 50-50% was irradiated for 60 seconds (Figure 9), with an initial temperature of 26 °C and a final temperature of 140 °C, with a temperature increase of 114 °C, as seen in the Table 1, a rapid increase in temperature. Although at 25 seconds there is a sudden temperature increase associated with interaction between microwaves and chemical properties of oil, as shown in Figure 12 FTIR.
Oil samples irradiated for 30 to 60 seconds
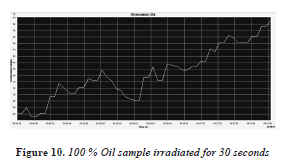
The sample of 100% oil was irradiated for 30 seconds, with an initial temperature of 25 ° C and a final temperature of 53 °C, with a temperature increase of 28 °C, shown in the Table 1. Shown in Figure 10 shows that 100% oil also has a substantial absorption of the microwave.
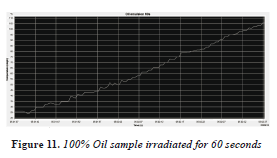
The second sample of 100% oil was irradiated for 60 seconds, with an initial temperature of 26 °C and a final temperature of 105 °C, with a temperature increase of 79 shown in Table 1. It can be observed in Figure 11 that shows the 100% sample also has considerable energy absorption, but not as much as the sample of 50-50% oil-water because the water accelerates energy absorption thereby causing greater polarization and thus further warming.
Spectra de FTIR (Fourier Transform Infrared Spectroscopy)
The oil was characterized by FTIR before being irradiated, and subsequently after 30 and 60 seconds of it being irradiated:
- Crude: Samples of crude from Constitutions Tamaulipas field were characterized before being irradiated.
- Oil-water: two characterizations were performed on samples of 50ml of crude oil which were irradiated for 30 and 60 seconds respectively.
- Crude: two characterizations were performed on 50ml samples of Oil-Water (50-50), which were irradiated for 30 and 60 seconds respectively.
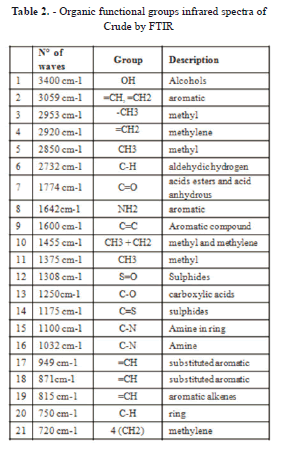
The main functional groups of absorption resins and asphaltenes are qualitatively identical. To facilitate the study of the results of this analysis, the spectrum is divided into four regions that can be interpreted as follows:
Group I: hydrogen valence vibrations (small) in the region of 3100-2700 cm-1. Group II: Vibrations valence double or partially double bonds, this region of 1900-1350 cm-1. Group III: dubbing vibrations out of the plane. Valence vibrations low energy region between 1000-710 cm-1. Group IV: Region valence vibrations of single and double bonds very strongly coupled, a region is observed in the range of 1350-1000 cm-1 band as a matter of oxygen valency vibrations. In the spectra obtained the following functional groups can be identified:
In accordance with the intensity changes of the absorption bands after interaction with microwave radiation shown in Figure 12, the following can be summarized:
- An increase in the content of aromatic structures, manifested in higher band intensity in regions 1600, 3040, 870, 815 and 750 cm-1. This may also relate to an increase in the degree of hydrogen substitution in aromatic structures.
- An increase in the content of aliphatic and alicyclic (naphthenic) groups mainly at the expense of the CH2 group, as indicated by the increased intensity of the bands in the 2930, 2860, and 1470 cm-1 regions and of the doublet at 730 -720 cm-1. A slight decrease in the number of CH3 groups is indicated by weakening of the band in the 1380 cm-1 region and a decrease in the "shoulder" in the 2950 cm-1 region
- A significant increase pf the OH functional group band at 3400 cm-1 and in the region of 1774 cm-1 corresponding to C = O. This is attributed to the recovery of polar compounds solubilized in the added water, such as alcohols, esters, acids and acid anhydride.
- An increase in the bands belonging to the region covered by 1350-1000 cm-1, corresponding to an increase of asphaltenes recovered during microwave irradiation, because of the added water.
4. CONCLUSIONS
Using the temperature module with the infrared sensor the temperature could be measured in real time, without the need to enter any sensor within the sample, as this would contaminate the oil sample or damage the sensor. Using this module the signal representative of the temperature could be visualized and recorded to be studied later.
The magnetron control module could be programed for the time or time intervals that the samples are to be irradiated using the graphical interface of , and to realize an emergency stop if the temperature rises above the limits established and so control the microwave heating process.
The crude oil, after being irradiated with microwaves, qualitatively retains the functional groups that it is compose of, there are only quantitative changes that are assumed to be due to the recovery of soluble compounds in injection water.
The increased intensity of the OH group (3400 cm-1) and C = O (1774 cm-1) confirms the recovery of soluble polar compounds in the injection water.
Asphaltenes, the main emulsion stabilizers present in the injection water are recovered. Microwave radiation, breaks the film of surfactant present in the water.
The spectra show that the time corresponding to 30 seconds irradiation, gave better results recovery of dissolved hydrocarbons (Figure 12). Establishing the lower irradiation time corresponds to low amount of energy required in the destabilization of the emulsion and therefore energy savings in the process.
ACKNOWLEDGEMENTS
The authors thank the company Pemex Exploration and Production for allowing sample collection in the Tamaulipas-Constituciones field.
REFERENCES
[1] Nour A. Emulsion Stability and Microwave Demulsification of Crude Oil Emulsion: Emulsion characterization, mechanisms of microwave heating technology, chemical demulsification, Lambert Academic Publishing, 2011, p. 25. [ Links ] [2] Flynn J. The Nalco Water Handbook, 2009, p. 813. [ Links ] [3] Klaila W. Method and Apparatus for Controlling Fluency of High Viscosity Hydrocarbon Fluids, 1978, U.S. Patent 4,067,683. [ Links ] [4] Wolf N. Use of Microwave Radiation in Separating Emulsions and Dispersions of Hydrocarbons and Water, 1986, U.S. Patent 4,582,629. [ Links ] [5] Countinho R. Method for the Microwave Treatment of Water-In-Oil Emulsions, 2010, U.S. Patent 7,705,058 B2. [ Links ]