Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.183 Medellín Jan./Feb. 2014
https://doi.org/10.15446/dyna.v81n183.34382
http://dx.doi.org/10.15446/dyna.v81n183.34382
ANAEROBIC DIGESTION OF FIQUE BAGASSE: AN ENERGY ALTERNATIVE
DIGESTION ANAEROBIA DEL BAGAZO DE FIQUE: UNA ALTERNATIVA ENERGÉTICA
HUMBERTO ESCALANTE H.
Ingeniero Químico PhD, Profesor Escuela de Ingeniería Química, Universidad Industrial de Santander, escala@uis.edu.co
CAROLINA GUZMÁN L.
Bacterióloga y Laboratorista Clínico, Profesora Escuela de Bacteriología y Laboratorio Clínico, Universidad Industrial de Santander, caguzlun@uis.edu.co
LILIANA CASTRO M.
Ingeniera Química PhD, Escuela de Ingeniería Química, UniversidadIndustrial de Santander, lcastrousta@gmail.com
Received for review October 26th, 2012, accepted September 9th, 2013, final version October 28th, 2013
ABSTRACT: In Colombia the agro-industrial process of fique generates approximately 20 800 kg of waste / ha planted, consisting of bagasse and juice. These wastes are discarded over soil and water generating a problem of environmental pollution. The fique bagasse (FB) has a calorific value of 3 297.91 kcal / kg, high concentrations of cellulose, hemicellulose and C / N ratio that make it appropriate for biogas production. However, the presence of lignin in the FB requires specific microbial consortia for its degradation. Therefore, in this research the biogas production from FB on a laboratory scale was studied through the Anaerobic Digestion (AD) process using a consortium of ruminal fluid (RF) and pig manure (PM). A methane production of 0.35 m3 CH4/kg volatile solids (VS) added during two weeks, equivalent to 1.38 kWh/kg VS added, indicated that FB is an attractive residual to be used as a source of renewable energy.
Key words: Anaerobic digestion, fique bagasse, inoculums, lignocellulosic waste, ruminal fluid.
RESUMEN: En Colombia, el procesamiento agroindustrial de fique genera aproximadamente 20 800 kg de residuos/ha sembrada que corresponden a. jugo y bagazo. Estos residuos son descartados al ambiente generando problemas de contaminación. El bagazo de fique tiene un valor calorífico de 3 297.91 kcal/kg, altas concentraciones de celulosa, hemicelulosa y una relación C/N favorable para tratar este residuo mediante conversión anaerobia. Sin embargo, la presencia de lignina en el bagazo hace que se requiera un consorcio microbiano específico para llevar a cabo la degradación. En este trabajo se estudio la producción de biogás a partir del bagazo de fique, empleando como inóculo una mezcla de líquido ruminal y lodo estiércol de cerdo. Se alcanzó una producción de metano de 0.35 m3CH4/kg Sólidos Volátiles (SV) adicionados durante quince días de digestión, equivalente a 1.38 kWh/kg SV adicionado, indicando que el bagazo de fique es un residuo atractivo para ser usado como fuente de energía renovable.
Palabras clave: Digestión anaerobia, bagazo de fique, inóculos, residuo lignocelulósico, líquido ruminal.
1. INTRODUCTION
Agricultural residues are an alternative source of energy that reduces the depletion of fossil fuels [1]. The molecular structure of this residual biomass is responsible for their energy content which varies between 3000 and 3500 kcal / kg for lignocellulosic wastes, and from 2000 to 2500 kcal / kg for urban waste [2]. The agro industrial process of fique (Agave family) generates 15000 tons of waste (bagasse) per hectare. Bagasse is being left on soil and thrown in rivers causing environmental pollution problems. Waste from the fique process have been evaluated for the production of pharmaceutical active compounds (hecogenin and tigogenin), surfactants, bioinsecticides, paper and fique fiber reinforced materials [3].
Due to the physicochemical composition of fique bagasse (FB), this residue is considered as a lignocellulosic waste biomass. [4].
Actually, one the most important impacts of renewable energy is the anaerobic digestion process from different substrates. The wastes´s physico chemical characteristics condition the biomethane potential.
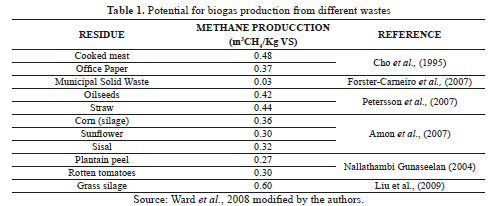
The Table 1 shows the yields values of 0.03 and 0.48 m3 CH4/kg VS added for urban municipal solid and cooked meat wastes, respectively.
Wastes with composition similar to Fique Bagasse, such as sisal, maize silage and grass silage, reached high biogas production, with yields of 0.24 m3 CH4/kg VS added, 0.36 m3 CH4/kg VS added and 0.6 m3 CH4/kg VS, respectively [1, 5]
In terms of methane yields, the Cattail aquatic plant reached a conversion of 66% from volatile solids, using ruminal fluid as inoculums [6]. Sisal and corn digestion attain methane values of 60% v/v [5, 7]. Whey, barley and rice residues showed high biomethane potential with values of 501, 229 and 195 L CH4/kg VS, respectively [8].
The high carbon content in FB makes it a potential substrate for methane production by anaerobic bioconversion systems [9]. Therefore, the aim of this research was to evaluate the production of biogas through anaerobic digestion on a laboratory scale, using a consortium of bacteria from ruminal fluid and pig manure.
Anaerobic digestion takes place in four stages: a) hydrolysis b) acidogenesis c) acetogenesis d) methanogenesis. These stages are carried out by microbial consortia formed from different populations of microorganisms. The products generated in each stage are the nourishment of another [10].
In hydrolysis, the organic matter composed of complex molecules must be broken to simpler compounds. The microorganisms involved in this stage produce acetic acid-carbon compounds, fatty acids and other organic polycarbonate compounds. In this way, carbohydrates are converted into simple sugars, fats into glycerol and fatty acids and proteins are hydrolysed to peptides and amino acids, releasing carbon dioxide and hydrogen [11]. At the acidogenesis stage, the monosaccharides produced are converted into organic acids of acetate, propionate, butyrate, valerate type, CO2 and H2. In acetogenesis, acetate, H2 and CO2 are generated, mostly. In methanogenesis, the methanogenic consortiums convert acetate into methane and CO2, mainly [12, 13].
The methane production depends on its hydrolytic activity (HA) and specific methanogenic activity (SMA). The HA indicates the inherent ability of a microbial population to degrade carbon and it is quantified as the specific rate of substrate consumption [14]. The SMA refers to the ability of the microbial biomass to convert organic matter into methane and it is expressed as the mass of substrate in terms of chemical oxygen demand (COD) that is converted into methane per biomass unit per unit of time (gCOD-CH4/g volatile suspended solids- VSS / day) [15].
Physico-chemical composition of FB indicates that these residues are composed of complex polymers such as cellulose, hemicelluloses and lignin. Therefore, FB digestion requires a specialized hydrolytic consortium. Different microbial consortiums have been used in biogas production from lignocellulosic materials, such as anaerobic sludge from primary wastewater treatment plants, ruminal fluid, pig manure or cattle manure, compost, and pure cultures of microorganisms [16, 17].
Previous studies showed that during anaerobic digestion from fique bagasse, the mixture Ruminal Liquid (RL) and Pig Waste Sludge (PWS), as consortia, showed high hydrolytic and methanogenic activities and the best biomethane potential.
During the anaerobic digestion process, the organic matter is converted into soluble fractions, which can be expressed as total reducing sugar (TRS), total volatile fatty acids (TVA) and cumulative methane volume.
The aim of this research, was to describe anaerobic digestion from fique bagasse, used as inoculum the mixture ruminal liquid and pig manure sludge, through evolution of total reducing sugar, total volatile fatty acids and cumulative volume methane.
2. MATERIALS AND METHODS
2.1. Substrate
FB, as substrate, was collected in a fique processing plant located in Santander -Colombia. FB chemical composition was evaluated by: total alkalinity (TA), concentration of total solids (TS), volatile solids (VS), volatile fatty acids (VFA), carbon / nitrogen ratio (C / N), cellulose, hemicellulose and lignin according to procedures established by Van Soest and the Standard Methods for Examination of Water and Wastewater [18, 19].
2.2. Inoculum
A mixture of 1:1 ruminal fluid (RF) and pig manure sludge (PMS) was used in the bioproduction process. RF was obtained from bovine stomachs collected in a livestock processing plant. PMS was collected from pig septic tanks. The inoculums physicochemical composition was evaluated according to protocols established by the Standard Methods for Examination of Water and Wastewater [18].
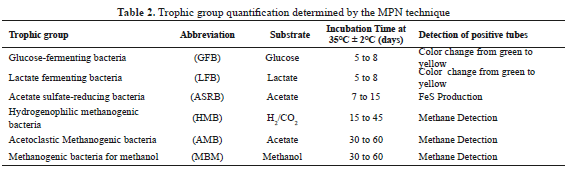
The microbiological characterization quantify the major microbial groups present in the inoculum and it was carried out using the technique of Most Probable Number (MPN) according to protocols established [15]. Serial dilutions were made from the mixture RF-PMS. Each dilution were inoculated in five hungate tubes, additionally, five un-inoculated tubes were considered as control. The tests were performed in a CO2 atmosphere to ensure anaerobic conditions. A positive result was identified according to the characteristics of each trophic group (Table 2). MPN values were calculated using the Mac Grady statistical table.
2.3. AD process for FB
Methane production from FB using RF-PMS was carried out in 0.5 L reactors, with an operating volume of 0.35 L. substrate / inoculum ratio of 1 g VS / g VS was used. The operation time was 15 days, at 39 ± 2°C. The concentration of total reducing sugars (TRS), volatile fatty acids (VFA), biogas volume and the percentage of methane produced were considered as variables response.
TRS concentration was determined according to the colorimetric method of dinitrosalicylic acid, using a GENESYS 20 Thermo Spectronics spectrophotometer at a wavelength of 540 nm [20]. VFA concentration was quantified according to the titration procedure [21]. Methane volume was measured by the alkaline shift method [22] at standard conditions and the quality of biogas produced was determined by a PGD3-IR Status Scientific Controls infrared gas detector. All fermentations were performed in duplicate. Experimental result was analyzed with standard deviation.
3. RESULTS AND DISCUSSION
3.1. Characterization of FB
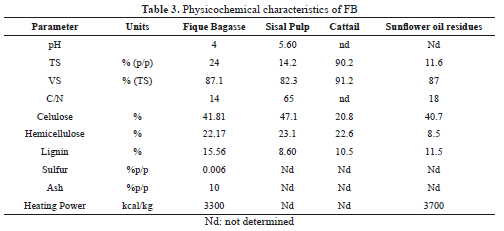
FB has similar physicochemical characteristics to sisal waste, cattail and sunflower oil residues (Table 3), all of them with high potential for biogas production [6, 23, 24].
FB has an acidic pH that could inhibit the start of AD process. However, the inoculum's buffering capacity regulates this inhibitory effect. According to the concentration of VS, cellulose and hemicelluloses content, FB has the capacity to produce methane and it is coherent with the biodegradability test in sisal [25].
The C/N content varies with the type of waste and causes inhibition in an inappropriate range. A C/N optimal range of 15 to 25 has been recommended for microbial growth. As examples, the co-digestion of onion and digested sludge has a value of 15; mixtures of corn crops with the sludge reach ratios between 15 and 18, and 21 in adverse operational conditions [13]. In this study FB has a C/N of 14.
In energy terms, olive waste has a calorific value of 4240 kcal / kg and reached the maximum biogas production of 54.26 l / l olive residue containing 83% methane [26, 27]. Sunflower oil residues has a calorific value of 3700 with productions of 0.20 L CH4/kg VS [24]. FB has a high calorific value, 3000 kcal / kg, which can be exploited for energy production, this value corresponds to agricultural biomass with the low content of sulfur and ash.
3.2. Physicochemical and microbiological characterization of the inoculums
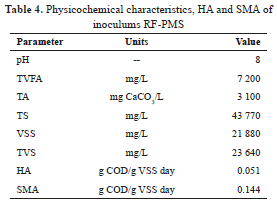
RF-PMS characterization is shown in Table 4. Values obtain from this inoculums confirm its application in AD process in terms of pH: 8.0 TVFA: 3100 mg/L and VSS: 21880 mg/L, among others parameters [28].
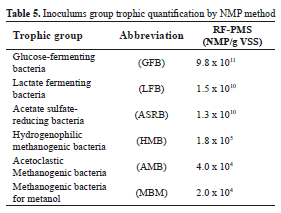
Microbial distribution of populations in RF-PMS is shown in Table 5. High levels of GFB, LFB and ASRB confirm the enzyme activity required for AD starting up (hydrolytic and acidogenic stages). The last group guarantees acetate metabolism since its ability to grow using this substrate of the incomplete oxidation of ethanol [29]. HBM, AMB and MBM concentrations between 104 and 105 NMP/g VSS maintain a partial pressure of hydrogen at a level that allows syntrophic degradation of ethanol and propionate [30, 31]. The percentage distribution of hydrogenophilic methanogenic archaea group (40%) is responsible for methane production and shown a symbiotic balance between the trophics groups.
3.3. TRS and VFA variation in AD from FB
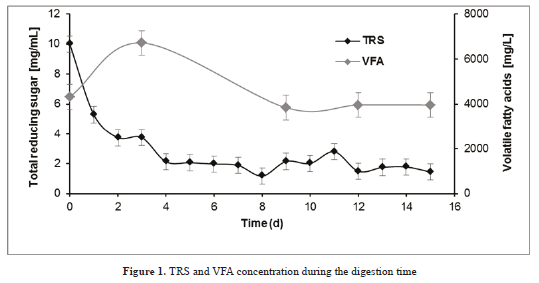
TRS are soluble compounds, easily metabolized by microorganisms, which allow the AD first stage to take place [24]. The high concentration of TRS at the beginning encourages the process to start (Figure 1). The rapid consumption of sugars, until the fourth day, is consistent with microorganisms metabolism in the hydrolysis and acidogenesis stages and shows its enzymatic capacity [26]. The TRS concentration was kept constant during the fermentation.
During VFA production, a simultaneous TRS consumption was observed. From the eighth day, VFA concentration remained constant (from 4000 to 4320 mg VFA / L) and avoids inhibition by acidification in the reactor. These results are consistent with VFA and pH values from other studies. For example, the variation of pH for biomethane potential of maize in a batch test, ranged from 7.2 to 8.0, similar results were obtained with fruit/vegetable with maximum values before inhibition of a pH of 7.8 and 7800 mg/l of VFA [7,33].
3.4. Methane production from FB
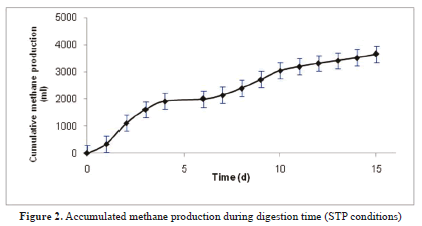
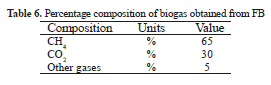
Methane production, for the first two weeks, was 3.6 L (Figure 2) and confirms the AD success using FB as an organic substrate. Yields values obtained were 0.45m3 CH4/kg VS and are comparable with the AD of grass, corn and agro-industrial wastes (0.40, 0.32 and 0.32; respectively) [8, 23, 34]. The percentage composition of the biogas produced from FB (Table 6), is corroborated with research about anaerobic digestion from sisal experiments, the methane production reached was above 50% [5]. These results indicate that FB can be considered as a viable alternative for recovering energy in the form of biogas with 60-65% methane content.
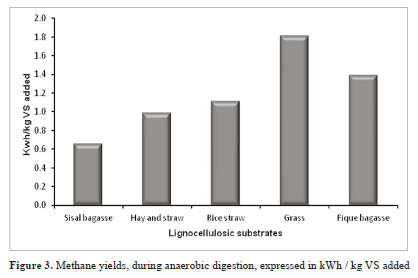
In comparison with other lignocelullosic wastes, FB is one of the most efficient biomasses in terms of electrical energy (Figure 3) [5, 8, 35].
4. CONCLUSIONS
Anaerobic Digestion of FB, as the lignocelullosic substrate, produces 1.38 kwh/kgVS using a mixture of ruminal fluid-pig sludge manure with high hydrolytic activity and specific methanogenic activity potential. Anaerobic digestion of fique bagasse is an alternative, not only as a real source of energy but also it contributes to reduce the environmental contamination.
ACKNOWLEDGEMENTS
The authors thank the Universidad Industrial de Santander, Ministerio de Agricultura y Desarrollo Rural y al Departamento de Ciencia y Tecnología de Colombia.
REFERENCES
[1] Ward A J., Hobbs P J., Holliman, PJ., Jones, D. L., "Optimisation of the anaerobic digestion of agricultural resources". Bioresource Technol, 99(17), pp.7928-7940, 2008. [ Links ] [2] Crotti, C. and Milera, S., "Implementación del modelo cropsyst para la simulación del rendimiento del cultivo de maíz en una región argentina". Centro de Investigación observación y monitoreo territorial y ambiental, pp. 2-4, 2006. [ Links ] [3] Ministerio de Agricultura y Desarrollo Rural; CADEFIQUE; CORPOICA. Guía Ambiental del Subsector Fiquero, Colombia, pp. 43-53, 2006. [ Links ] [4] Mata-Alvarez, J., Macé, S. and Llabrés, P., "Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives". Bioresource Technol., 74(1), pp. 3-16, 2000. [ Links ] [5] Mshandete, A., Kivaisi, A., Mugassa, R. and Bo, M.,"Anaerobic batch co-digestion of sisal pulp and fish wastes". Bioresource Technol, 95(1), pp. 19-24, 2004. [ Links ] [6] Zhen-Hu, H. and Han-Qing, Y.,"Anaerobic digestion of cattail by rumen cultures". Waste manage, 26(11), pp. 1222-1228, 2006. [ Links ] [7] Raposo, F., Banks C.J., Siegert, I., Heaven, S. and Borja, R., "Influence of inoculum to substrate ratio on the biochemical methane potential of maize in batch tests". Process biochem, 41(6), 2006, pp. 444-1450. [ Links ] [8] Dinuccio, E., Balsari, P. Gioelli, F. and Menardo, S., "Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses". Bioresource Technol, 101(10), pp. 3780-3783, 2010. [ Links ] [9] Barrera, P., Salas, X., Castro, L., Ortiz, C. and Escalante, H., "Estudio preliminar de la bioproducción de metano a partir de los residuos del proceso del beneficio del bagazo de fique". Revista Ion, 22(1), pp. 21-25, 2009. [ Links ] [10] Kumar Khanal Samir. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications. Editorial W. Blackwell, USA, pp. 43-53, 2008. [ Links ] [11] Appels Lise; Baeyens Jan; Degrève Jan; Dewil Raf. "Principles and potential of the anaerobic digestion of waste-activated sludge". Prog energ combust, 34(6), pp. 755-781, 2008. [ Links ] [12] Mata Alvarez, J., Biomethanization of the Organic Fraction of Municipal Solid Wastes, Editorial IWA, España, pp.5-20, 2002. [ Links ] [13] Yebo, Li., Stephen, Y. P. and Jiying, Z., "Solid-state anaerobic digestion for methane production from organic waste". Renew sust energ rev, 15(1), pp. 821-826, 2011. [ Links ] [14] Hu, Z-H., Wang, G. and Yu, H-Q., "Anaerobic degradation of cellulose by rumen microorganisms at various pH values". Biochem Eng J, 21(1), pp. 59-62, 2004. [ Links ] [15] Díaz, M. C., Espitia, S. E. y Molina, F., Digestión Anaerobia: Una aproximación a la tecnología. Editorial Universidad Nacional de Colombia UNIBIBLOS. Colombia, pp. 43-45, 2002. [ Links ] [16] O´Sullivan Cathryn, A., Burrell C. Paul; Clarke William, P., Blackall, Linda L., "Comparison of cellulose solubilisation rates in rumen and landfill leachate inoculated reactors". Bioresource Technol, 97(18), pp. 2356-2363, 2006. [ Links ] [17] Forster-Carneiro, T., Pérez, M., Romero, L.I. and Sales, D., "Dry-thermophilic anaerobic digestion of organic fraction of the municipal solid waste: Focusing on the inoculum sources". Bioresource Technol, 98(17), pp. 3195-3203, 2007. [ Links ] [18] American Public Health Association, APHA. Standard Methods for the Examination of Water and Wastewater. Edition 20th, USA, 1998. [ Links ] [19] Goering H.K. and Van Soest, P.J., Forage. Fiber Analyses (Apparatus, Reagents, Procedures and some Applications. Agricultural Handbook 379, ARS-USDA, United States of America, 1970. [ Links ] [20] Miller, G., "Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar". Anal Chem, 31(3), pp.426-428, 1959. [ Links ] [21] Anderson, G.K. and Yang, G., "Determination of bicarbonate and total volatile acid concentration in anaerobic digesters using a simple titration". Water Environ Rese 64, pp. 53-59, 1992. [ Links ] [22] Angelidaki, I., Alves, M., Bolzonella, D., Borzacconi, L., Campos, J., Guwy, A. J., Kalyuzhnyi, S., Jenicek, P. and Van Lier, J. B. "Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays". Water Sci Technol., 59 (5), pp. 927-934, 2009. [ Links ] [23] Mshandete, A., Bjornsson. L., Kivaisi, K.A. and Rubindamayugib, S.T., Mattiassona Bo. "Enhancement of anaerobic batch digestion of sisal pulp waste by mesophilic aerobic pre-treatment". Water Res., 39 (8), pp. 1569-1575,2005. [ Links ] [24] Galí, A., Benabdallah, T., Astals, S. and Mata-Alvarez, J., "Modified version of ADM1 model for agro-waste application". Bioresource Technol., 100 (1), pp. 2783-2790, 2009. [ Links ] [25] Kivaisi, K. A. and Rubindamayugi, M.S.T., "The potential of agro-industrial residues for production of biogas and electricity in Tanzania" Renew Energ., 9 (1-4), pp. 917-921,1996. [ Links ] [26] Fernández, J., "Los residuos de las agroindustrias como biocombustibles sólidos (I)". Vida rural. pp. 14-18, 2006. [ Links ] [27] Fezzani, B. and Ben, CR., "Two-phase anaerobic co-digestion of olive mill wastes in semi-continuous digesters at mesophilic temperature" Bioresource Technol., 101 (6), pp. 1628-1634, 2010. [ Links ] [28] Quintero, M., Castro, L., Ortiz, C., Guzmán, C. and Escalante, H., "Enhancement of starting up anaerobic digestion of lignocellulosic substrate: fique´s bagasse as an example". Bioresource Technology. Vol. 108, pp. 8-13, 2012. [ Links ] [29] García, M.C., Díaz Báez, M.C., "Evaluación de la toxicidad de un efluente cervecero mediante ensayos de inhibición de la actividad metanogénica". Rev. Colomb. Biotecnol., 5(2), pp. 23-31, 2003. [ Links ] [30] Valdez-Vazquez Idania; Poggi-Varaldo. "Hydrogen production by fermentative consortia". Renew Sust Energ Rev., 13(5),pp.1000-1013, 2009. [ Links ] [31] Gerardi, M., The Microbiology of Anaerobic Digesters. Editorial Wiley Interscience. Estados Unidos, pp. 81-117, 2003. [ Links ] [32] De La Rubia, M.A., Raposo, F., Rincón, B. and Rincón, Borja R., "Evaluation of the hydrolytic-acidogenic step of a two-stage mesophilic anaerobic digestion process of sunflower oil cake". Bioresource Technol., 100(18), pp. 4133-4138. 2009, [ Links ] [33] Dinsdale, R. M., Premier, G.C., Hawkes, F.R. and Hawkes, D., "Two-stage anaerobic co-digestion of waste activated sludge and fruit/vegetable waste using inclined tubular digesters". Bioresource Technol., 72(2), pp. 159-168, 2000. [ Links ] [34] Mata-Alvarez, J., Dosta, J., Macé, S. and Astals, S., "Codigestion of solid wastes: A review of its uses and perspectives including modeling". Crit Rev Biotechnol., 31(2), pp. 99-111, 2010. [ Links ] [35] Álvarez, J., Otero, L. and Lema, J., "A Methodology for Optimising Composition for Anaerobic Co-digestion of Agro-industrial Wastes". Bioresource Technology. Vol. 101, No. 1, pp. 1153-1158. 2010 [ Links ]