Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.183 Medellín Jan./Feb. 2014
https://doi.org/10.15446/dyna.v81n183.41568
http://dx.doi.org/10.15446/dyna.v81n183.41568
IRON ORE SINTERING PART 2. QUALITY INDICES AND PRODUCTIVITY
SINTERIZACIÓN DE MINERALES DE HIERRO PARTE 2. ÍNDICES DE CALIDAD Y PRODUCTIVIDAD
JAVIER MOCHÓN
Ph. D., Centro Nacional de Investigaciones Metalúrgicas CSIC-CENIM (Spain), jmochon@cenim.csic.es
ALEJANDRO CORES
Ph. D., Centro Nacional de Investigaciones Metalúrgicas CSIC-CENIM (Spain), alcores@cenim.csic.es
ÍÑIGO RUIZ-BUSTINZA
Ph. D., Centro Nacional de Investigaciones Metalúrgicas CSIC-CENIM (Spain), irbustinza@cenim.csic.es
LUIS FELIPE VERDEJA
Ph. D. Escuela Técnica Superior de Ingenieros de Minas, Oviedo (Spain), lfv@etsimo.uniovi.es
JOSÉ IGNACIO ROBLA
Ph. D., Centro Nacional de Investigaciones Metalúrgicas CSIC-CENIM (Spain), jrobla@cenim.csic.es
FERNANDO GARCIA-CARCEDO
Ph. D., Centro Nacional de Investigaciones Metalúrgicas CSIC-CENIM (Spain), g.carcedo@cenim.csic.es
Received for review March 14th, 2013, accepted August 27th, 2013, final version September, 6th, 2013
ABSTRACT: Sinter plants have to process mineral mixes in order to obtain sinter of a suitable composition and quality to be loaded into the blast furnace. For this purpose a series of parameters need to be taken into account, such as the nature and composition of each component of the mineral mix and the conditions of the manufacturing process. Sinter is subjected to in-depth characterisation in terms of chemical and granulometric analysis, determination of the mineral phases in its structure and of quality indices such as reducibility, low temperature degradation, reduction degradation, and tumbler strength. It is also important to operate sinter plants with high productivity and to ensure a uniform sinter composition and quality so as to facilitate the steady state operation of the blast furnace.
Key words: Sintering, Quality indices, Productivity, Blast furnace
RESUMEN: Las plantas de sinterización tienen que procesar mezclas de mineral con el fin de obtener sinterizado de una composición y calidad adecuada para ser cargados en el alto horno. Para este fin se deben de tener en cuenta una serie de parámetros, tales como la naturaleza y la composición de los componentes de la mezcla de minerales así como de las condiciones del proceso de fabricación. El sinter es sometido a una caracterización en profundidad; análisis químico y granulométrico, determinación de las fases minerales en su estructura e índices de calidad, tales como reducibilidad, la degradación de la baja temperatura, la reducción de la degradación, y la resistencia - tumbler. También es importante que las plantas de sinterización funcionen con una alta productividad, garantizando una composición y calidad uniforme con el fin de facilitar la operación estable del horno alto.
Palabras clave: Sinterización, Índices de Calidad, Productividad, Horno Alto

1. INTRODUCTION
Improvements in the blast furnace process are achieved by technological improvements and a stable supply of high quality iron ores and sinters [1]. However, high quality iron ore resources are being depleted due to the heavy demand, and thus it is necessary to continue improving sintering technology in order to use lower quality iron ores in the raw mix. The blast furnace demands sinter with high strength, a low RDI, high RI, low fines content, good average calibrated sinter size and little variation in chemical composition in order to operate in a steady state regime. Efforts are being made to supply blast furnace operators with high quality sinter. Sinter quality control, by means of adequate sintering, is important in order to operate blast furnaces at a low fuel rate and stable operating rate.
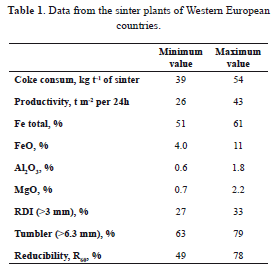
In Western Europe, 9 countries operate 36 sinter plants which in 2004 manufactured 100.8 million tons of sinter. Table 1 sets out data on coke consumption, productivity, and sinter composition and quality indices in European sinter plants [2]. The figures show a low Al2O3 content, acceptable RDI values, and a large difference between the minimum and maximum parameter values.
2. QUALITY INDICES
The ongoing improvement of blast furnace operation is largely due to improvements in sinter quality. The blast furnace demands sinter with a high cold strength, low reduction degradation index and high reducibility index, in a very narrow band of chemistry variation, with the lowest possible fines content and a good average size.
2.1. Chemical composition
The chemical and structural composition are very important in sinter, and it is good for them to be stable so that both primary and final slags possess adequate characteristics in terms of softening and melting temperatures, liquid temperature and viscosity for the stable operation of the blast furnace.
It is important to have a high iron content, low gangue content, and basicity of the order of 1.6-2.1. Sinter reducibility, and sinter quality in general, improves with a higher level of hematite than magnetite, and its structure improves with a higher level of primary or residual hematite and ferrites than secondary or precipitated hematite.
2.1.1. FeO sinter
The FeO content is an important control parameter in the sinter plant. When the chemical composition of an ore mix is fixed, FeO can provide an indication of sintering conditions, in particular the coke rate [3]. A 2% increase in the FeO content in sinter has been found to lower (improve) the RDI by 8 points. However, a higher FeO content negatively affects reducibility. It is important to find an optimum FeO content in order to improve the RDI without altering other sinter properties [4].
2.1.2. Al2O3 sinter
The most harmful effect of alumina is to worsen the sinter RDI, which increases as the alumina content rises. Industrial experience with the blast furnace shows that within a 10-10.5% CaO content range an increase of 0.1% in the alumina content raises the RDI by 2 points [4]. The strength and quality of sinter deteriorate as the alumina content rises. Alumina promotes the formation of SFCA, which is beneficial for sinter strength, but the strength of the ore components is lower, since a high alumina content in their lattice has been reported to be the main cause of the observed lower strength [5,6]. Alumina increases the viscosity of the primary melt that forms during the sintering process, leading to a weaker sinter structure with more interconnected irregular pores [7].
Sinter reducibility is determined by the chemical and mineralogical composition and by the pore structure. Due to the complexity of the effects of alumina on each of these factors, consideration of how alumina affects reducibility has produced contradictory results [8]. In work carried out in a sinter pot loaded with 65 kg of ore mixes with different alumina contents, an increase in the alumina content from 2 to 5.5% raised the sinter RI from 58 to 64% [9].
2.1.3. MgO sinter
MgO provides for an optimum blast furnace slag condition in terms of both good flowability and desulphurisation. It can be added to the blast furnace as raw flux in the form of dolomite or dunite, or as sinter. The addition of MgO to the raw mix improves the RDI, because MgO stabilises magnetite and thus decreases the hematite content, giving rise to less stress in the sinter during the hematite to magnetite reduction in the blast furnace stack [10].
It has been determined that replacing CaO with MgO in the form of dolomite for basicities of 1.6-1.9 leads to a slight reduction in sinter strength, reducibility and productivity [11,12]. In research carried out in a sinter pot with 65 kg of raw mix, the MgO content of four manufactured sinters was increased from 1.4 to 2.6% by the addition of dolomite to the mix. The iron ore used presents a low MgO content (0.01%) and a high Al2O3 content (2.99%). It was seen that raising the MgO content in the sinter, from 1.4 to 2.6%, increased the FeO content and decreased productivity and the RI, RDI and TI indices [13].
2.1.4. CaO sinter
CaO combines with the iron oxides to form compounds with a low melting point that favour the formation of the primary melt, a minimum level of which is needed in order to manufacture a strong sinter. These compounds are: Fe2O3·CaO (1205 °C) and FeO·CaO (1120 °C). The properties of the melt formed during sintering determine the structure of the bonding phases originated in the sinter. The melt properties in the moments prior to solidification depend to a large extent on the chemical composition of the fines layer adhered to the granules and the assimilation of nucleus particles [14-16].
2.1.5. SiO2 sinter
Silica combines with FeO and CaO to form compounds with a low melting point that favour the formation of the primary melt: FeO·SiO2 (1180 °C), 2FeO·SiO2 (1205 °C), and FeO·SiO2·CaO (1223 °C). Increasing the silica content and the basicity of the adherent fines causes the primary melt formation temperature to drop, which is favourable for the subsequent assimilation reaction at the liquid-solid interface between the fines and the nucleus particles [16,17].
2.2. Granulometric distribution
After being tipped from the pallets in the sintering machine, the sinter is hot screened. Its granulometric distribution is an important process parameter. The 12-35 mm fraction is sent directly to the blast furnace hoppers, the larger fraction is crushed to obtain smaller sized fractions, and the <5 mm fraction (return fines) is recycled to the sinter plant hoppers.
For the good operation of the process, it is important to keep a balance between the generation and recycling of return fines [2]:

The sinter is screened and each of the resulting fractions is weighed: >40 mm; 40-20 mm; 20-12 mm; 12-5 mm and <5 mm. The combined weight of all the fractions comprises the total cake weight. The useful sinter is the total cake minus the return fines generated (>5 mm fraction). The average grain size is calculated as a function of the kg of sinter corresponding to each fraction, and can vary over a broad interval between 25 and 45 mm.
To avoid excessive crushing of the product sinter as it leaves the strand, some modifications have been made to the crusher. Kawasaki Steel Corporation (KSC) has improved productivity at its Kimitsu [18] and Chiba [19] plants (Japan) by using a smaller screen aperture for return fines. Kobe Steel has reduced the screen aperture for return fines in its Kakogawa plant (Japan) [20] from 5 to 4.5 mm, and has replaced the preskip 5 mm screens in the blast furnace with 3.5 and 4 mm screens. Changes to screen sizes and types have also been adopted at Chiba [19], as a consequence of which productivity has risen by 2%, and a rate of 80 kg fine sinter t-1 of HM can be used at No. 6 BF.
2.3. Reducibility index (RI)
Reducibility is an important characteristic of sinters which measures the ability to transfer oxygen during reduction in the blast furnace stack, giving an idea of fuel consumption needs in the furnace. The porosity and structure of the sinters and their mineral phases are intimately related with their reducibility. A heterogeneous structure is more reducible than a homogeneous structure [21-23]. It is also possible to predict reducibility behaviour from the concentration of each phase present [24]. The reducibility of mineral phases in decreasing order is:

Hematite and magnetite are rapidly reduced to wustite (FeO), but the rates differ for subsequent reduction to metallic iron [25]. From hematite, wustite is quickly and homogenously reduced, although some wustite is surrounded by metal. From magnetite, the reduction is topochemical, following the sequence Fe3O4 → FeO → Fe, and almost all the wustite grains are surrounded by metallic iron, which delays the subsequent reaction. The reducibility of SFCA may be related with their morphology, porosity and whether or not they are coated with glass [26-28]. Acicular ferrite (<10 µm) formed at low temperature (<1300 °C) is more reducible, while columnar ferrite (>10 µm) formed at high temperature (>1300 °C, possibly coated with glass) is less reducible. Primary hematite is more reducible than secondary hematite because of its intrinsic porosity.
It has been researched how the mineralogy and porosity of iron ores affect reducibility [29,30]. Sinters have been manufactured in the laboratory (bench scale) and in a pilot plant and the relationship between porosity, reducibility and the tumbler index is determined. Higher porosity leads to greater reducibility, and the sinters with the largest surface area (open pores) present a more fragile structure and lower TI. Researchers have also investigated the behaviour of chlorine and alkalis in the blast furnace and their effect on sinter properties during reduction [31]. Studies have been carried out in the laboratory and in No. 5 BF operated by Roheisengesellshaft Saar (ROGESA) at Dillinger (Germany). The laboratory tests have shown that despite some differences the effects of chlorine, which combines to form KCl and NaCl, and alkalines on sinter are on the whole quite similar. Sinter reduction tests at up to 1100 °C show that the presence of alkalis favours the reduction of hematite to magnetite, due to the catalytic action of the alkali. The presence of chlorine compounds is unfavourable, as they are deposited on the sinter surface and inhibit its reduction. The presence of alkalis leads to an increase in the sinter stress, due to an increase in the reduction of hematite to magnetite, and cracks form which increase abrasion. By inhibiting the reduction reaction, chlorine compounds assure less abrasion up to 700 °C. At higher temperatures, the reduction reaction increases, with the corresponding rise in abrasion.
2.4. Low temperature degradation (LTD) index
The degradation of sinter is determined by the Low Temperature Degradation Index (LTD) and the Reduction Degradation Index (RDI). Sinter degradation during reduction at low temperature is determined by the dynamic LTD test, which is carried out at 600°C [32]. Degradation is originated, to a certain extent, in the transformation that takes place during the reduction of hematite to magnetite, accompanied by an increase in volume, giving rise to the presence of structural stresses in the sinter [33]. The degradation of sinter in the blast furnace occurs during reduction in the low temperature zone, and has a harmful effect on the burden strength in the furnace, with the consequent loss of permeability to reducing gases and an increase in coke consumption [34].
In research involving the addition of magnetite fines in a raw mix for sintering, a coke saving of 0.43% was seen for each 1% increase in magnetite in the raw mix, due to the fact that when hematite ore is replaced by magnetite fines, the bed temperature rises as a result of the exothermic oxidation reaction of magnetite to hematite [35-37]. An increase of 5.1% was also seen in the LTD index for each 1% increase in hematite in the raw mix during the oxidation of magnetite, which is transformed into g-Fe2O3 with the same cubic spinel lattice structure as magnetite. The TI and RI indices do not undergo any noticeable change when hematite is replaced by magnetite.
2.5. Reduction degradation index (RDI)
Sinter degradation during reduction at low temperature is more usually determined by the RDI static test [38], which is carried out at 550 °C. Low values are desirable for this index. The RDI is a very important parameter that is used as a reference in all sintering work and serves to predict the sinter's degradation behaviour in the lower part of the blast furnace stack.
Secondary hematite, also known as skeletal rhombohedral hematite, is the main cause of a poor sinter RDI. This is based on the frequent observation of cracks around the narrow neck regions of such hematite. On the other hand, it has been suggested that the cracks which form due to the volumetric change that accompanies the transformation of the crystalline phase from hematite to magnetite are responsible for the reduction degradation of the sinter [39]. Studies involving TEM, XRD and Vickers indentation tests have shown that secondary hematite is the most harmful sinter component for RDI [40]. Secondary hematite generally contains dissolved impurities like Al2O3, TiO2 and MnO which raise the stress in magnetite by distorting the lattice. This magnetite forms during hematite reduction at 550 °C in the blast furnace.
Using XRD and dilatometry it has been found that the sinter structure depends on the maximum temperature reached in the bed, and that secondary hematite is present at higher temperatures [41]. Secondary hematite forms as a result of recrystallisation during the sintering of primary hematite. At lower temperatures a greater proportion of primary hematite (residual hematite) remains in the sinter composition. It has been observed that Al2O3 tends to be concentrated in the secondary hematite phase when the primary hematite → secondary hematite transformation takes place. Once again it is seen that an increase in the Al2O3 and TiO2 concentration in sinter is harmful for the RDI. Continuing with this research [42], it was determined that the presence of a solid dissolution of Al2O3 and TiO2 in hematite originates a 4% volume expansion during the reduction of hematite to magnetite at 550 °C, and causes distortion of the crystalline lattice of these phases and an increase in the magnitude of lattice stresses in the magnetite formed. The presence of cracks in the sinter structure after reduction at 550 °C are more frequent in the regions with a higher secondary hematite content, and harmful for the RDI, as has been noted.
The production rate and RDI have been researched in a sinter plant using neural networks [43]. The model considered 55 parameters and analysed a group of 695 RDI values recorded over a 3-year period. It was found that the production rate and the RDI depended on the same variables. A strong relationship was seen between the RDI and the outdoor ambient temperature at the plant. The RDI was also strongly dependent on the Ti content in the sinter, even when this was only very small. No relationship with alumina was found due to its low content (0.5%) and scarce variation in the tested period. The model found the coke ratio in the sinter mix to be the most important control variable with regard to the RDI.
To improve the operation of its No. 3 and No. 4 BFs at Fukuyama, Nippon Kokan Keihin (NKK) lowered the SiO2 content in the sinters in its No. 4 and No. 5 SPs from 4.8 to 4.2%, taking into account pulverised coal injection rates of the order of 170 kg t-1 of HM. This led to an improvement in furnace permeability and reducibility, but worsened the RDI44. A relation was thus found between bed permeability and the RDI. NKK developed a totally new kind of optical fibre radiation thermometer named FIMT (fibre in metallic tube) which measures the temperature close to the tap hole immediately after tapping. With the combined actions of lowering the silica content in the sinter and improved melt temperature control, it has managed to lower the silica content in pig iron from 0.3 to 0.2% [44]. It has been reported that a 6% improvement in sinter RDI would lower the blast furnace coke rate by 14 kg t-1 of HM and increase blast furnace productivity by 3% [45].
2.6. Tumbler index (TI)
The cold strength of sinter is determined by the tumbler test [46], and depends on the strength of each individual ore component, the strength of the bonding matrix components and the ore composition. This test determines the size reduction due to impact and abrasion of the sinters during their handling, transportation, and in the blast furnace process. Studies of the fracture strength of several mineral phases have allowed the following order to be established: primary (or residual) hematite > secondary hematite > magnetite > ferrites. Cold mechanical strength is directly related with the tendency for fines to form during transportation and handling between the sinter machine and the blast furnace throat.
The sinter strength depends to a large extent on the properties of the matrix formed by vitreous glass, silicates, olivines and ferrites. Vitreous glass presents a high degree of stress: the allotropic transformation, which starts at 697 °C, from β-2CaO·SiO2 to g-2CaO·SiO2 is accompanied by a change in volume that causes the sinter strength to decrease. Ferrites have been identified as a strong bonding material that improves sinter strength [47,48]. The TI of sinter is dependent on critical flaws in the sinter and their propagation through sinter particles. Flaws are unavoidable because the different minerals and phases precipitate out of the melt at different times during the cooling cycle, and changes in volume almost always accompany the transformation of a liquid into a solid [49].
A research has been carried out in pilot plant and industrial scale, to enhance the strength of a sinter with high iron (58.8%) and low silica (4.38%) content [50]. By adding of serpentine and burnt lime into the ore mix to be sinterized and with a deeper sinter bed, there is a significant increase in the amount of magnesium ferrite and SFCA, which is associated with improved in sinter strength.
2.7. Sinter porosity
Sinter porosity is an important parameter that significantly affects its properties, in particular its reduction behaviour. Porosity (P) is calculated by determining the real density (dR) and apparent density (dA) of sinters before and after being subjected to the reducibility test:

Sinters experience a strong increase in porosity after undergoing the reducibility test.
In research carried out with hematite and goethite [51] ores, the changes caused to the initial pore structure during reduction tests at 550 and 950 °C were analysed. It was seen that the pore diameter needs to be larger than 0.01 mm for the reducing gas to have sufficient access to the pores to satisfactorily reduce the sinter. When the micropores coalesced to pores of a size of more than 1 to 5 mm, the specific surface area of the sinter decreased and so did its reduction [52].
Research has shown that eliminating the coalescence of micropores and increasing the number of small pores makes it possible to increase the surface area of the sinter and obtain a substantial improvement in its reducibility. Ferrites stabilise the micropores and lead to a rise in porosity, thus achieving higher reducibility [53,54]. The ferrite decomposition reaction to produce magnetite and silicates can be achieved at high temperature in a reducing atmosphere, and is the most important reaction to decrease sinter porosity.
Besides the increase in sinter porosity after being subjected to the reducibility test, there is also an increase in volume originated during the transformation of hexagonal hematite into cubic magnetite. The increase in volume that takes place due to this transformation is 25%.
The crystal structure of magnetite (Fe3O4) is of the spinel type, with a = 8.38 Å. It has a close-packed cubic lattice of O2- ions with the smaller Fe2+ and Fe3+ ions distributed in the interstices. Hematite (a-Fe2O3) is of rhombohedral corundum type (a = 5.42 Å and x = 55° 14'). The O2- ions are arranged in a close-packed hexagonal lattice and two thirds of the octahedral interstices are occupied by Fe3+ ions. The oxide has a small oxygen deficit, probably because of O2- vacancies, but possibly also due to iron ions in additional interstitial positions [55].
2.8. Sinter structure
Given the diversity of the mineralogical components that comprise the raw mix, as well as the heterogeneity of the mix, it is understandable that the sinter structure will also be complex, being formed mainly by grains of iron oxide and calcium ferrites bonded by a gangue matrix [56-58]. The ferrites, whose amount increases with the basicity index, are easily reduced, and by increasing the mechanical toughness of sinter to certain levels are considered very useful components. The ferrites are SFCA type and form by a solid-liquid reaction between hematite and the Fe2O3·CaO melt, with the subsequent assimilation of SiO2 and Al2O3 in the melt. The gangue is composed of calcium, iron and magnesium silicates that are difficult to reduce, and come to form part of the slag in the blast furnace.
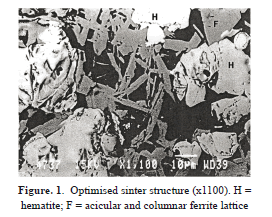
The structure and composition of sinter includes the presence of primary hematite (non-assimilated or residual), secondary hematite (precipitated), primary magnetite (non-assimilated or residual), secondary magnetite (precipitated) and ferrites as major phases, along with a smaller amount of gangue. There is sufficient porosity to favour the reducibility of the sinter, including micropores in many cases. The optimum structure for reducibility is formed by a nucleus of primary hematite surrounded by a lattice of acicular ferrites, as shown in Figure 1.
Anglo Research (South Africa) has developed a methodology for the characterisation of iron ore sinters and iron ore slime through the use of its QUESCAN, which uses EDS, SEM and BSE instruments. QUESCAN model results are based on an order of magnitude (approximately 10000 times) greater number of analysis points than those obtained by point counting [59,60].
3. PRODUCTIVITY
In a sinter plant, the onus is on achieving high productivity. This is done by assuring good bed permeability, and for this it is essential to optimise the granulation process. Moreover, for high sinter productivity it is necessary to maximise the sinter output. Various factors can influence output, such as: horizontal and vertical uniformity in the sinter bed, sinter bonding strength, crushing of product sinter, and selection of return fines screen aperture [19,20].
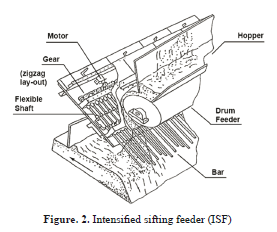
Non-uniform sintering usually results in part of the bed being more friable and can lead to high fines production. Where there is a lack of vertical uniformity, it is often necessary to increase the coke content in the top part of the bed. This is possible by segregation of the feed using devices such as an intensified sifting feeder (ISF) or a slit bar chute [61] (Fig. 2). Horizontal uniformity is improved by using multi-segment gates on the roll feeder outlet. The problem is most serious near the pallet walls where the air flow is highest. This can be reduced by compacting the top of the bed close to the side walls or installing a dead bar grate near the wall [62]. KSC has installed a new burner line to improve ignition at its Chiba works (Japan), thereby achieving more uniform sintering and consequently an increase in productivity [18]. ArcelorMittal has installed a more compact ignition hopper at its Fos-sur-Mer works (France), with 3 rows of burners under the hood [63]. Productivity has increased by 1 t m-2 per 24h as a result of more intense ignition, or because a larger surface area of the strand is now available for sintering.
In some plants higher production has been achieved by increasing the bed depth, generally together with a reduction in the strand speed. For this type of operation high permeability is essential and some improvements to granulation may be necessary, such as the addition of (more) lime. At its Burns Harbor works (USA), Bethlehem Steel raised the bed depth from 406 mm to 635 mm and reduced the strand speed from 2.4 to 2 m min-1, achieving a productivity increase of almost 30% [64]. KSC increased the bed depth at its Mizushima works (Japan) from 530 to 700 mm, achieving a 6% gain in productivity [65].
At its Kakogawa works (Japan), Kobe Steel enriches the air with oxygen [20]. Oxygen is injected below the hood that covers a large part of the strand, after the ignition hood. This improves coke consumption, with the result of operating with a narrower heating zone and a higher flame front speed. It is possible to improve production by 1 t h-1 with the use of a flow of 500 Nm3 oxygen. Many plants produce sinter with a 1.5-3% MgO content by adding dolomite, serpentine or olivine in the feed [64]. Higher productivity is achieved with olivine and serpentine than with dolomite, a fact that may be attributed to the harmful effect of dolomite on sinter strength, and thus on output.
In research carried out at BHP Billington's Newcastle Technology Centre, productivity was seen to be the main challenge facing users of pisolitic ore [65]. It was widely observed that incorporating pisolitic ores in blends composed predominantly of Australian and Brazilian hematite ores causes sinter plant productivity to drop. The reason for this is a reduction in bed permeability caused by excessive melt formation. To improve productivity, water addition during granulation can be increased in order to compensate the fact that porous pisolite ore particles absorb a significant part of the added granulation water and thus reduce the amount of free water available on their surfaces for inter-particle adhesion, leading to a deterioration in granulation efficiency.
Research has been carried out in a laboratory pot grate, varying the MgO content in the raw material from 1.40 to 2.60% [66]. Dolomite and dunite are used as fluxes to add magnesia. Increasing the MgO content in the sinter mix means a higher temperature is required for melt formation, and the highly fluxed composition with MgO acts as a refractory phase, raising heat consumption and reducing productivity. On the other hand, it has been found that increasing the MgO content improves the RDI, due to the drop in hematite and ferrite phases and the rise in the magnetite phase, which presents lower degradation. Tata Steel published data recorded between April 1994 and March 2001 in its No. 2 SP at Jamshedpur (India) [67]. Raising the MgO content (range 1.75-3.25%) caused the plant productivity to decrease. The TI increased, but it was considered that for MgO contents of more than 4% the TI would decrease due to the formation of a vitreous matrix that exhibits a high degree of stress and a low formation of bonding phases. In contrast with the preceding work [66], it was seen that an increase in MgO also raised the RDI. This variation may be due to differences between experimental conditions and actual plant data.
REFERENCES
[1] Cores, A., Verdeja, L. F., Ferreira, S., Ruiz-Bustinza, Í. and Mochón, J., Dyna, vol. 80, pp. 152- 171, 2013. [ Links ] [2] Cores, A., Babich, A., Muñiz, M., Ferreira, S. and Mochón, J., ISIJ Int., vol. 50, pp. 1089-1098, 2010. [ Links ] [3] Loo, C. E., Proc. 2nd Int. Cong. on Sci. and Tech. Ironmaking. ISS Ironmaking Conf. Proc., 57, pp. 1229-1316, 1998. [ Links ] [4] Kumar, C. U., Ramana, R. V., Ali, S., Das, A. K., Kumar, A., De, A. K. and Mukherjee, T., ,Tata Search, pp. 20-25, 1995. [ Links ] [5] Sinha, M. and Ramna, R. V., ISIJ Int., 49, pp. 719-721, 2009. [ Links ] [6] Ostwald, J. and Pepper, M. D., BHP Tech. Bull., 26, pp. 46-48, 1982. [ Links ] [7] Loo, C. E. and Leung, W., ISIJ Int., 43, pp. 1393-1402, 2003. [ Links ] [8] De, S., Gupta, S. and Chatterjee, A., SEAISI Q., 21, pp. 35-46, 1992. [ Links ] [9] Umadevi, T., Deodar, A. V., Mahapatra, P. C., Prabhu, M. and Ranjan, M., Steel Res. Int., 80, pp. 686-692, 2009. [ Links ] [10] Lu, L., Holmes, R. J. and Manuel, J. R., ISIJ Int., 47, pp. 349-358, 2007. [ Links ] [11] Panigrahy, S.C., Riguad, M. A. J. and Dilewijins, J., Steel Res., 56, pp. 35-41, 1985. [ Links ] [12] Panigrahy, S. C., Verstraeten, P. and Dilewijins, J., Ironmaking Steelmaking, 11, pp. 17-22, 1984. [ Links ] [13] Umadevi, T., Nelson, K., Mahapatra, P. C., Prabhu, M. and Ranjan, M., Ironmaking Steelmaking, 36, pp. 515-520, 2009. [ Links ] [14] Napoleão, A., Pinheiro, P., Caparoli, L., Oliveira, D. L. A., Fujikawa, L. and Vervenee, R., Proc. 3rd Int. Conf. on Sci. and Tech. of Ironmaking, VDEh, Düsseldorf, Germany, pp. 127-132, 2003. [ Links ] [15] Hsieh, L. H. and Whiteman, J. A., ISIJ Int., 33, pp. 462-473, 1993. [ Links ] [16] Debrincat, D., Loo, C. E. and Hutchens, M. F., ISIJ Int., 44, pp. 1308-1317, 2004. [ Links ] [17] Bakker, T. and Heerema, R. H., ISS Ironmaking Conf. Proc., 55, pp. 487-491, 1996. [ Links ] [18] Saito, G., Motizuki, M., Amakawa, K., Kamiko, Y., Harada, T. and Yamada, H., Trans. Iron Steel Inst. Jpn., 28, B90, 1988. [ Links ] [19] Obata, H., Takahashi, H., Nakamura, M., Natsumi, T. and Komamura, K., ISIJ Int., 31, pp. 478-486, 1991. [ Links ] [20] Shibuta, K., Kuwano, K., Ito, R., Awaji, M., Ano, K. and Sugiyama, T., ISS Ironmaking Conf. Proc., 49, pp. 623-628, 1990. [ Links ] [21] Bhagat, R. P., Chattoraj, U. S. and Sil, S. K., ISIJ, 46, pp. 1728-1730, 2006. [ Links ] [22] Hessien, M. M., Kashiwaya, Y., Ishii. K., Nasr, M. I. and El-Geassy, A. A., Ironmaking Steelmaking, , 35, pp. 191-204, 2008. [ Links ] [23] Panigrahy, S. C., Rigaud, M. A. J. and Hegedus, G. S., Ironmaking Steelmaking, , 21, pp. 353-361, 1994. [ Links ] [24] Sakamato, N., Fukuyo, H., Iwata, Y. and Niyashita, T., Proc. 12th Mc Master Symp. on "Burden design for the blast furnace", Mc Master University, Hamilton, ON, Canada, pp. 137-150, 1984. [ Links ] [25] Maeda, T. and Ono, T., Trans. Iron Steel Inst. Jpn., 25, pp. 1191-1193, 1985. [ Links ] [26] Scarlett, N. V. Y., Pownceby, M. I., Madsen, I. C. and Christensen, A. N., Metal. Mater. Trans. B, , 35B, pp. 929-936, 2004. [ Links ] [27] Maeda, T., Nishioka, K., Nakashima, K. and Shimizu, M., ISIJ Int., 44, pp. 2046-2051, 2004. [ Links ] [28] Wang, S., Gao, W. and Kong, L., Ironmaking Steelmaking, , 25, pp. 296-301, 1998. [ Links ] [29] Busby, N. J. and Fray, T. A. T. "Pyrometallurgie 87". The Institution of Mining and Metallurgy in assotiation with The Institute of Metals. London, United Kingdom, pp. 141-166, 1987. [ Links ] [30] Busby, N. J., Fray, T. A. T. and Goldring, D. C., Ironmaking Steelmaking, 21, pp. 229-236, 1994. [ Links ] [31] Lectard, E., Hess, E. and Lin, R., Proc. 3rd Int. Conf. on Sci. and Tech. of Ironmaking, VDEh, Düsseldorf, Germany, pp. 424-429, 2003. [ Links ] [32] ISO Proposal, ISO/TC-102/SC-2/286-E, ISO, Geneve, Switzerland, 3rd Draft, 1974. [ Links ] [33] Roller, P. W., Trans Iron Steel Inst. Jpn., 26, pp. 834-835, 1986. [ Links ] [34] Kortman, H. A. and Burghardt, A., "Agglomeration 77", Ed. K. V. S. Sastry, Iron and Steel Soc. of AIME, New York, USA, pp. 219-231, 1977. [ Links ] [35] Panigrahy, S. C., Rigaud, M. A. J. and Hegedus, G. S., Ironmaking Steelmaking, 21, pp. 235-261, 1994. [ Links ] [36] Panigrahy, S. C., Rigaud, M. A. J., Chong, S. P. and Chanda, B., ISS Ironmaking Conf. Proc., 53, pp. 523-531, 1994. [ Links ] [37] Button, R. A. and Lundh, P. A., Ironmaking Steelmaking, 16, pp. 151-164, 1989. [ Links ] [38] ISO/TC-102/SC-3/285-E. A method for determining the low temperature disintegration behaviour of iron ores, pellets and sinters by cold tumbling after static reduction, ISO Proposal, ISO, Geneve, Switzerland, 3rd Draft, 1974. [ Links ] [39] Dawson, P. R., Ironmaking Steelmaking, 20, pp. 137-143, 1993. [ Links ] [40] Shigaki, I., Sawada, M. and Gennai, N., Trans. Iron Steel Inst. Jpn, 26, pp. 503-511, 1986. [ Links ] [41] Pimenta, H. P. and Seshadri, V., Ironmaking Steelmaking, 29, pp. 169-174, 2002. [ Links ] [42] Pimenta, H. P. and Seshadri, V., Ironmaking Steelmaking, 29, pp. 175-179, 2002. [ Links ] [43] Kinnunen, K. and Laitinen, P., Rev. Mètall. CIT, 102, pp. 361-371, 2005. [ Links ] [44] Rokugawa, S., Noda, H., Ichikawa, K., Sakamoto, N., Sato, H. and Watanabe, T., Rev. Mètall. CIT, 96, pp. 1181-1190, 1999. [ Links ] [45] Murty, C., De, A., Chatterjee, A. and Rao, V. S., Tata Search, pp. 7-13, 1994. [ Links ] [46] "Standard method for tumbler test for iron ores, pellets and sinters". Designation: E279-69 (Reapproved 1979), ASTM, Pittsburg, PA, USA, 1980. [ Links ] [47] Dartnell, J. J., Iron Steel Inst., 207, pp. 282-292, 1969. [ Links ] [48] Barrie, D. J., Carmichael, I. F. and Kinloch, E. D. J., Iron Steel Inst., 207, pp. 563-569, 1969. [ Links ] [49] Lwamba, E. and Garbers-Craig, A. M., J. S. Afr. Inst. Min. Met., 108, pp. 293-300, 2008. [ Links ] [50] Zhu, D., Qiu, G., Fan, X., Hu, Y., Xiau, L., Tian, F., Chen, W. and Clout, J., Proc. 3rd Int. Conf. on Sci. and Tech. of Ironmaking, VDEh, Düsseldorf, Germany, pp. 133-137, 2003. [ Links ] [51] Restrepo, O. J., Vásquez, C. F. and Bastamente M. O., Dyna, vol. 75, pp. 163-170, 2008 [ Links ] [52] Bristow, N. J., Goss, J. and Waters, A. G., Iron Steelmaker, 18, No. 9, pp. 61-79, 1991. [ Links ] [53] Bristow, N. J. and Loo, C. E., ISIJ Int., 32, pp. 819-828, 1992. [ Links ] [54] Bhagat, R. P., Chattoraj, U. S. and Sil, S. K., ISIJ Int., 46, 1728-1730, 2006. [ Links ] [55] Edström, J. O., "Symposium on Sinter". Special Report No. 53, The Iron and Steel Institute, London, United Kingdom, pp. 10-25, 1955. [ Links ] [56] Egundebi, G. O. and Whiteman, J. A., Ironmaking Steelmaking, 16, pp. 379-385, 1989. [ Links ] [57] Ahsan, S. N., Mukherjee, T. and Whiteman, J. A., Ironmaking Steelmaking, 10, pp. 54-64, 1983. [ Links ] [58] Mukherjee, T. and Whiteman, J. A., Ironmaking Steelmaking, 12, pp. 151-155, 1985. [ Links ] [59] Tonetic, I. and Dippenaar, A., Miner. Engin., 24, pp.1258-1263, 2011. [ Links ] [60] Banerjee, A. and Mukherjee, A. K., ISIJ Int., 51, pp. 1392-1395, 2011. [ Links ] [61] Inazumi, T., Fujimoto, M. and Sato, K., Proc. 6th Int. Iron and Steel Cong., Nagoya, Japan, ISIJ, pp. 110-117, 1990. [ Links ] [62] Nakajima, R., Kurosawa, S., Fukuyo, H. and Yamaoka, Y., Proc. 6th Int. Iron and Steel Cong., Nagoya, Japan, ISIJ, pp. 163-170, 1990. [ Links ] [63] Huguet, C., Russo, P. and Soland, J., ISS Ironmaking Conf. Proc., 50, pp. 549-554, 1991. [ Links ] [64] Stubel, J. F. and Durko, D. P., ISS Ironmaking Conf. Proc., 47, pp. 657-669, 1988. [ Links ] [65] Matsumoto, K., Matsuda, K., Nakajima, Y. and Akizuki, H., ISS Ironmaking Conf. Proc., 47, pp. 613-619, 1988. [ Links ] [66] Umadevi, T., Roy, A. K., Mahapatra, P. C., Prabhu, M. and Ranjan, M., Steel Research Int., 80, pp. 800-807, 2009. [ Links ] [67] Yadav, U. S., Pandey, B. D., Das, B. K. and Jena, D. N., Ironmaking Steelmaking, 29, pp. 91-95, 2002. [ Links ]