Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.183 Medellín jan./fev. 2014
https://doi.org/10.15446/dyna.v81n183.36896
http://dx.doi.org/10.15446/dyna.v81n183.36896
TWO-DIMENSIONAL COMPUTATIONAL MODELING OF THE ELECTROKINETIC REMEDIATION OF A COPPER-CONTAMINATED SOIL PART I: MODEL VALIDATION
MODELACIÓN COMPUTACIONAL EN DOS DIMENSIONES DE LA REMEDIACIÓN ELECTROCINETICA DE UN SUELO CONTAMINADO CON COBRE PARTE I: VALIDACIÓN DEL MODELO
VIRGILIO RUBIO-NIEBLAS
MSc. Instituto de Ingeniería, Universidad Autónoma de Baja California. Mexicali, México, virgilio62@gmail.com
MANUEL PEREZ-TELLO
PhD. Departamento de Ingeniería Química y Metalurgia, Universidad de Sonora. Hermosillo, México, mperezt@iq.uson.mx
RICHARD A. JACOB
Independent consultant. Houston, Texas, U.S.A., rajacobs@gmail.com
RONALDO HERRERA-URBINA
PhD. Departamento de Ingeniería Química y Metalurgia, Universidad de Sonora, Hermosillo, México, rherrera@guaymas.uson.mx
SERGIO A. MORENO-ZAZUETA
PhD. Departamento de Ingeniería Civil y Minas, Universidad de Sonora. Hermosillo, México, alan@dicym.uson.mx
Received for review January 26th, 2013, accepted October 8th, 2013, final version November, 12th, 2013
ABSTRACT: A two-dimensional computational model for the electrokinetic remediation of copper from a contaminated soil is presented. The model is an adaptation of the original code developed by one of the authors, in which the chemical composition and transport of the copper species in solution were incorporated. The model includes electromigration, electroosmosis, ordinary diffusion, and convection transport mechanisms, it assumes local chemical equilibrium and unsteady state conditions. An artificially contaminated soil from Cananea, Mexico was used as an example. The model predictions were compared with experimental data collected in a laboratory, circular-shaped soil in which a central anode and four cathodes in the periphery were inserted. A reasonable agreement between the predicted and the experimental values was obtained in terms of the spatial distributions of pH and copper concentration. The potential applications of the computational model are discussed.
Key words: Electrokinetic remediation, copper-contaminated soil, mathematical model.
RESUMEN: Se presenta un modelo computacional en dos dimensiones para la eliminación electrocinética de cobre de un suelo contaminado. El modelo es una adaptación del código original desarrollado por uno de los autores, al cual se incorporaron la composición química y el transporte de las especies de cobre en solución. El modelo incluye los mecanismos de transporte por electromigración, electroósmosis, difusión ordinaria y convección, se supone equilibrio químico local y régimen no estacionario. Un suelo natural de Cananea, México se utilizó como ejemplo. Las predicciones del modelo se compararon con datos experimentales obtenidos a nivel laboratorio en un suelo circular con un ánodo central y cuatro cátodos insertados en la periferia. Las predicciones del modelo concordaron razonablemente bien con las distribuciones espaciales de pH y concentración de cobre. Se discuten las aplicaciones potenciales del modelo computacional.
Palabras clave: Electrocinética, modelo matemático.
1. INTRODUCTION
The contamination of soils by heavy metals is a current concern worldwide. Metallic contaminants are typically retained within the soil components, but may be released and transported to the aqueous phase in the pores and other soil locations depending on both weather conditions and the spatial distributions of pH and oxidizing potential within the soil [1]. The remediation of affected sites has been proposed by several techniques. The present work focuses on an extraction process usually referred to as electrokinetic remediation of soils [2-6]. In this process, the affected soil is water-flooded to promote the dissolution of contaminants as ionic species in solution. Next, a series of electrodes is inserted into the soil, and an electrical potential difference is applied. The electric field causes the motion of the ionic species towards the electrodes of opposite sign, where they react to form insoluble neutral compounds which precipitate rapidly. At the end of the process, the small volume of the contaminant-rich soil in the vicinity of the electrodes may be removed by mechanical techniques for further treatment and disposal. An alternative way of operation consists of injecting a continuous flow of fresh water to selected electrode wells while the electrical potential difference is applied. The bulk flow of water facilitates the transport of the contaminants through and out of the soil by convection. The electrokinetic remediation process is a novel technology which is currently under development. The literature shows a number of experimental studies [2-6] for different types of contaminants. Such a practical knowledge has been complemented with mathematical formulations [2,7-9] aimed at representing on a quantitative basis the relevant phenomena occurring in the system; namely, electroosmosis, electromigration, ordinary diffusion, and convection, which are responsible for the transport of the chemical species throughout the soil. Such phenomena occur simultaneously with homogeneous, liquid-phase chemical reactions, as well as the electrochemical decomposition of water at the surface of the electrodes. Adsorption of some of the species on the surface of the soil pores may also occur [2,8]. The present investigation was motivated by recent reports regarding soil contamination by copper in some areas of the city of Cananea, Mexico [10]. Based on the analysis of the soil samples [11], it was of interest to elucidate the potential of electrokinetic remediation as a candidate to treat such soil. Over the last years, significant advances on the elucidation of the mechanisms governing the electrokinetic remediation of soils have been made [2-9]. Whereas most of the literature has focused on one-dimensional systems in which one anode and one cathode are used, recent experimental works [12,13] have addressed two-dimensional systems in which a spatial distribution of electrodes is considered. From a theoretical standpoint, Jacobs and Probstein [9] reported on the first two-dimensional mathematical model for the electrokinetic remediation of soils. The model was verified by comparing its predictions with experimental data collected in a bed of kaolin particles. Recently, Vereda-Alonso et al. [14] reported on a simplified two-dimensional model in which the motion of the chemical species was mostly attributed to electromigration; thus, electroosmosis, diffusion and convection mechanisms were neglected. The model predictions showed good agreement with the experimental data collected in a square two-dimensional porous medium made up of kaolin particles. The present investigation is based on a previous study by Jacobs [15], who found that electrode arrangements in which the anodes are surrounded by a number of cathodes provide greater efficiencies than those in which the cathodes are surrounded by the anodes. To the best of the authors´ knowledge, such an electrode arrangement has yet not been studied experimentally in the literature. The goal of this investigation was twofold: (1) to test whether the original formulation by Jacobs and Probstein [9] was capable of predicting with reasonable accuracy the kinetics of electroremediation of an artificially contaminated soil, and (2) to analyze the main features of the process in a two-dimensional soil field by means of computer simulation. Parts I and II of this series are thus devoted to such goals, respectively.
2. MODEL FORMULATION
The goal of the mathematical formulation was to provide a quantitative representation of the relevant phenomena occurring during the electrokinetic remediation in a two-dimensional framework. The mathematical model developed by Jacobs and Probstein [9] was the starting point. Because a detailed description of the mathematical formulation was reported by the authors [9], only a brief description is presented here. The continuity equation for the i-th aqueous species within the porous medium is written as
![]()
where all the symbols are defined in the Nomenclature. The flux Ni includes the contributions by electroosmosis, electromigration, ordinary diffusion, and convection transport mechanisms. The numerical solution of Equation (1) is difficult by conventional finite-difference methods such as Runge-Kutta and related algorithms [16]. This is because the chemical reaction terms Ri are several orders of magnitude higher than the transport terms appearing on the left-hand side of Equation (1), which causes numerical instabilities. An alternative way of solving Equation (1) is by assuming local chemical equilibrium throughout the porous medium. This allows the specification of a new set of independent quantities Tk defined by
![]()
where aik is a stoichiometric coefficient representing the contribution of species i to the conserved quantity k, N is the total number of chemical species, and M is the number of chemical reactions occurring in the system. Symbols Tk are defined in such a way that they are not affected by the chemical reactions. Multiplying through Equation (1) by aik and taking the summation over all i values yields
![]()
Because the amount of every element is conserved throughout the chemical reactions, the right-hand side of Equation (3) is equal to zero. Further substitution of Equation (2) into Equation (3) yields, upon rearrangement
![]()
Because the right-hand side of Equation (4) does not contain chemical reaction terms, this equation may be solved by conventional finite-difference and finite-element methods. The numerical solution of Equation (4) thus provides the values of the conserved quantities Tk as functions of both time and position within the porous medium. Once the Tk values are known, the concentration of the individual species in the solution Ci may be computed from Equation (2) coupled to the chemical equilibrium relationships:
![]()
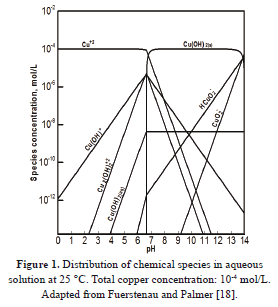
Equations (2) and (5) is a set of N nonlinear algebraic equations with N unknowns; namely, C1 through CN. In this study, the set of equations was solved by means of the multivariable Newton-Raphson algorithm [16]. The specification of the chemical system was computed according to the procedure described by Butler and Cogley [17], and was based on the equilibrium diagram for the copper-water system reported by Fuerstenau and Palmer [18]. The resulting diagram is shown in Figure 1.
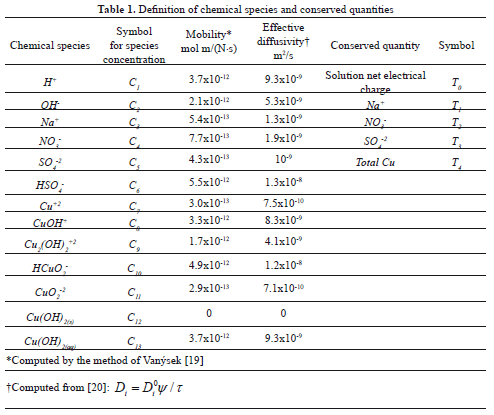
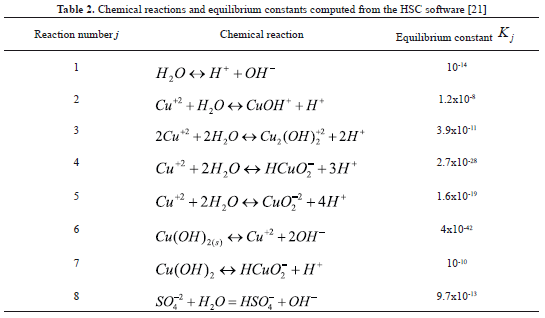
The resulting chemical system included: N = 13 chemical species and M = 5 conserved quantities, and is shown in Table 1. Also shown are the mobilities and effective diffusivities of the chemical species. Four continuity equations for the conserved quantities: T1 through T4 were solved within the porous medium. Quantity T0 is the net electrical charge of the aqueous solution, which must be zero at all times. Thus, it is a restriction for the local values of the species concentrations. Table 2 shows the chemical reactions considered in this study, as well as their equilibrium constants computed from the HSC software [21]. The specification of such reactions satisfies thermodynamic consistency. In addition to the homogeneous reactions occurring in the aqueous phase, the heterogeneous decomposition of water was assumed to occur at the anode surface: 2H2O(aq)→4H+(aq)+O2(g)+4e-. At the cathode surface, water was assumed to react according to: 2H2O(aq)+2e-→2OH-(aq)+H2(g). From a numerical standpoint, such reactions represent boundary conditions to Equation (4). The model also includes the equations to compute the density of total electric charge, the electrostatic potential, and the pressure distribution throughout the soil. Details of such equations and the overall numerical strategy are discussed elsewhere [9] and thus are not repeated here. For numerical purposes, the system boundaries were established beyond the vicinity of the electrode wells so that mass transfer across the boundaries was assumed to be zero. The model equations were solved in rectangular coordinates by means of a finite-element algorithm, and the results were analyzed within the framework of the Tecplot visualization software [22].
3. EXPERIMENTAL WORK
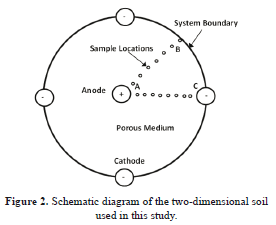
The goal of the experimental program was to provide data to validate the mathematical model described above. Figure 2 shows a schematic representation of the experimental set up used in this study. A circular, 0.4 m diameter, 0.35 m-height acrylic cell was used to contain a two-dimensional soil field. The cell included five graphite electrodes.
One electrode working as the anode was placed at the center of the arrangement, whereas four electrodes working as the active
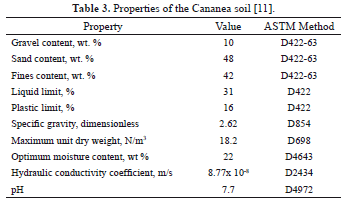
cathodes were placed at the border of the cell at right angles with respect to the center anode. The electrode wells consisted of five PVC cylindrical shells cut in half and glued to the inside of the acrylic cell. The half shells were perforated on their curved side to allow for the aqueous solution from the surrounding soil to flow inside the wells. The soil used in the experiments was from the copper mine at the city of Cananea, Mexico. This soil was free of copper contamination, it was previously sieved to eliminate particles larger than 0.635 cm (1/4 inch), and stored in plastic bags. Table 3 summarizes the properties of the soil after sieving [11]. According to the criteria established by the Unified Soil Classification System, this is a sandy clay soil. The unit dry weight of the soil was found to be 14.5 N/m3, with a porosity of 44.8%. The chemical composition of the soil was determined by atomic absorption spectroscopy (AAS). From this analysis, the following amounts in weight percent were determined [11]: 54.7% SiO2, 3.2% Fe2O3, 2.6% CaO, 2.2% K2O, 1.47% MgO, and 0.15% Mn2O3. The soil also contained Zn (122 mg/kg), elemental Cu (60 mg/kg) and Pb (35 mg/kg). The native copper present in the soil did not come from a contaminant source. Preliminary experiments with water showed that the copper initially present in the soil was motionless under the presence of the electric field. Because the native copper is not intended to be removed by electroremediation, this was a good experimental result. In a typical electrokinetic experiment, 2 kg of sieved soil was placed inside the acrylic cell and distributed uniformly by a systematic shaking procedure. The soil was artificially contaminated by flooding it with an aqueous solution of copper sulfate. The solution was prepared so that a pre specified copper concentration was obtained (100 and 600 mg/L). The solution was poured onto the soil bed until saturation, and the system was left at rest for two days covered with a plastic cover. A direct-current power source was used to generate the electric field. For a given initial concentration of copper in the soil, replicated experiments were carried out for 24, 48 and 72 hours. Before and after the experiments, 2 mL samples of the aqueous solution were collected with a plastic syringe at the sample locations shown in Figure 2. The samples were analyzed to determine the pH by a potentiometer, and the copper concentration by AAS. The sample locations were placed every 4 cm along two radial directions as shown in Figure 2. The first radial direction connected the surface of the central anode to the surface of the cathodes in the periphery (line AC), and is referred to as the anode-to-cathode distance. The second direction followed a path forming a 45-degree angle with respect to line AC, and will be referred to as anode-to-border distance (line AB). Five equally-spaced sampling locations along lines AC and AB were established. For line AC, sample locations 1 and 5 were placed at the surfaces of the anode and cathode, respectively. For line AB, sample location 1 was 2 cm away from the anode surface, whereas sample location 5 was set to match the surface of the acrylic cell border.
4. DISCUSSION OF RESULTS
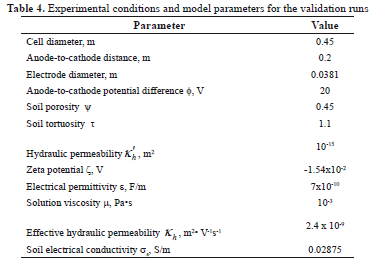
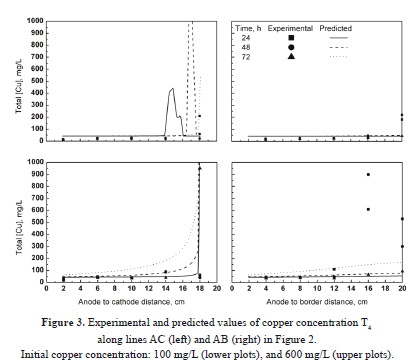
Table 4 shows the experimental conditions and model parameters used for the validation runs. Figure 3 shows the predicted and experimental values of total copper concentration T4 along lines AC (left plots) and AB (right plots) as functions of time and initial copper concentration in the soil (lower and upper plots). For a given initial concentration of copper, a comparison of left and right plots shows that copper species traveled faster along the AC direction than the AB direction, as the experimental values in the vicinity of the cathode (left plots) are higher than their respective values in the vicinity of the cell border (right plots). This was an expected result because the intensity of the electric field reaches its maximum along line AC, which in turn causes the metallic ions to move at the fastest rate. Similarly, for a given AC or AB direction, a comparison of lower and upper plots indicates that the higher the initial content of copper in the soil, the slower the motion of the copper species. As a result, more copper was left behind when 600 mg/L was used (upper plots) as compared to the experiments conducted with 100 mg/L of initial copper (lower plots). A general trend observed in Figure 3 consists of a sudden change in the experimental values of copper concentration within a few centimeters across the soil. This change was up to two orders of magnitude for samples collected 4 cm apart. In this paper, the location at which this phenomenon occurs is referred to as the reaction front. Along line AC, the reaction front is observed in the proximity of the cathode surface, whereas for line AB it occurred within 4 cm of the cell border. During the experiments, the reaction front was observed to move outwards as time progressed. The motion of the reaction front involves the formation of an isoelectric zone and is explained later in this paper.
As far as the mathematical model is concerned, the presence of an abrupt reaction front makes the prediction of the system characteristics difficult. Figure 3 shows that the concentration profiles computed by the model along line AC (left plots) are smooth compared to the experimental profiles. Also, the rate of transport of the copper species within the time frame of zero to 48 h was underpredicted. For 72 hours, the computed values agreed well with the experimental data. Along line AB (right plots), the calculated values showed a reasonable agreement with the experimental data, with the exception of the experiment conducted with 600 mg/L of initial copper, in which case the model overpredicted the rate of copper motion. Despite the complex behavior of the experimental system, the present model was capable of predicting the general trends observed in the experiments. Overall, the model predictions in the proximities of the central anode agreed well with the experimental data in all cases studied. The copper concentration in this region was typically less than 50 mg/L, whereas near the cathodes and the cell boundaries it increased by up to three orders of magnitude.
A relevant feature of the present formulation is its capability to predict the concentration peaks of copper within the soil, as shown in Figure 3. The peak in copper concentration occurs simultaneously with a sudden change in pH across the reaction front. This behavior was also observed experimentally.
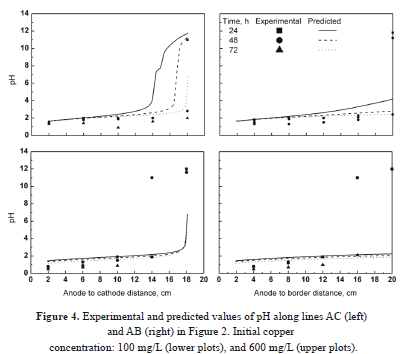
Figure 4 shows the predicted and the experimental values of pH for all cases studied. The trends are similar to those observed for copper concentration. Overall, the calculated values agreed well with the experimental values in the vicinity of the anode, whereas the prediction of the reaction front was less accurate. The behavior of the reaction front can be explained as follows. The electrolysis reactions cause significant changes in pH in the vicinity of the electrodes. At the reaction front, the Cu+2 ions react with OH- ions to form Cu(OH)2 according to reaction 6. Because Cu(OH)2 is a neutral molecule, both the solution and the soil in this area increase their electrical resistance. As a result, an isoelectric region appears, and the transport of all the ionic species in this area is hindered.
5. CONCLUDING REMARKS
The two-dimensional mathematical model developed by Jacobs and Probstein [9] was adapted to represent the electrokinetic remediation of an artificially copper-contaminated soil from the city of Cananea, Mexico. The model predictions showed reasonable agreement with experimental data collected in a laboratory cell in terms of copper concentration and pH. For the circular arrangement, the model predicted the formation of an isoelectric region which moves from the central anode towards the cathodes in the periphery. This region is characterized by the presence of high gradients of both copper concentration and pH.
An overall evaluation of the present formulation indicates that the phenomena occurring during the electrokinetic remediation of a natural soil are complex, and require further investigation from both experimental and theoretical perspectives. Despite these difficulties, the present formulation was capable of representing the main features of the process in a two-dimensional soil. It also clarified the potential of the electrokinetic remediation to treat a copper-contaminated soil. Investigation on both experimental and theoretical aspects of this process is currently in progress in this laboratory, and will be the subject of a future publication.
6. NOMENCLATURE

REFERENCES
[1] Hernán, S.L., González, L.M. y Espinosa, A., Modelación de elementos traza en el horizonte de suelos, plancha 170 (Vélez, Departamentos de Santander y Boyacá), (in spanish). Dyna, 156, pp. 157-164, 2008. [ Links ] [2] Shapiro, A.P., Renaud, P.C. and Probstein, R.F., Preliminary studies on the removal of chemical-species from saturated porous media by electroosmosis, Phys. Hydrodyn. 11(5-6), pp. 785-802, 1989. [ Links ] [3] Probstein, R.F. and Hicks, R.E., Removal of contaminants from soils by electric fields. Science, 260(5107), pp. 498-503, 1993. [ Links ] [4] Hamed, J., Acar, Y.B. and Gale, R.J., Pb(II) removal from kaolinite by electrokinetics. J. Geotech. Eng.-ASCE, 117(2), pp. 241-271, 1991. [ Links ] [5] Denisov, G., Hicks, R.E. and Probstein, R.F., On the kinetics of charged contaminant removal from soils using electric fields. J. Coll. Interface Sci., 178(1), pp. 309-323, 1996. [ Links ] [6] Page, M.M. and Page, C.L., Electroremediation of contaminated soils. J. Env. Eng.-ASCE, 128(3), pp. 208-219, 2002. [ Links ] [7] Shiba, S., Hirata, Y. and Seno, T., Mathematical model for hydraulically aided electrokinetic remediation of aquifer and removal of nonanionic copper. Eng. Geol., 77(3-4), pp. 305-315, 2005. [ Links ] [8] Jacobs, R.A., Sengun, M.Z., Hicks, R.E. and Probstein, R.F., Model and experiments on soil remediation by electric fields. J. Env. Sci. Health Part A-Environmental Science and Engineering & Toxic and Hazardous Substance Control, 29(9), pp. 1933-1955, 1994. [ Links ] [9] Jacobs, R.A. and Probstein, R.F., Two-dimensional modeling of electroremediation. AIChE J., 42(6), pp. 1685-1696, 1996. [ Links ] [10] Gómez-Álvarez, A., Meza, F.A., Villalba, A., Valenzuela, J., Ramírez, J. and Almendáriz, J., Estimation of potential pollution from mine tailings in the San Pedro river (1993-2005), Mexico-US border. Env. Geol. 57(7), pp. 1469-1479, 2009. [ Links ] [11] Moreno-Zazueta, S.A., Restauración por electroadsorción de suelos contaminados (In Spanish), [M.S. Thesis], Universidad de Sonora, Mexico, 1999. [ Links ] [12] Almeira, J., Peng, C. and Wang, Z., Effect of different electrode configurations on the migration of copper ions during the electrokinetic remediation process. Asia-Pac. J. Chem. Eng., 4(5), pp. 581-585, 2009. [ Links ] [13] Kim, D. H., Jo, S.U., Choi, J.H., Yang, J.S. and Baek, K., Hexagonal two dimensional electrokinetic systems for restoration of saline agricultural lands: a pilot study. Chem. Eng. J., 198-199, pp. 110-121, 2012. [ Links ] [14] Vereda-Alonso, C., Rodríguez-Maroto, J.M., García-Delgado, R.A., Gómez-Lahoz, C. and García-Herruzo, F., Two-dimensional model for soil electrokinetic remediation of heavy metals. Aplication to copper spiked kaolin. Chemosphere, 54, pp. 895-903, 2004. [ Links ] [15] Jacobs, A. R., Removal of contaminant material from a soil site. US Patent No. 5415744, 1995. [ Links ] [16] Carnahan, B., Luther, H.A. and Wilkes, J.O., Applied Numerical Methods, John Wiley & Sons, Inc., 1969. [ Links ] [17] Butler, J.N. and Cogley, D.R., Ionic equilibrium solubility and pH calculations, John Wiley & Sons, Inc., 1998. [ Links ] [18] Fuerstenau, M.C. and Palmer, B.R., Anionic flotation of oxides and silicates. In: Flotation - A. M. Gaudin Memorial Volume-Volume 1, (M.C. Fuerstenau, Editor), Chapter 7, American Institute of Mining, Metallurgical, and Petroleum Engineers Inc., New York, pp. 148-196, 1976. [ Links ] [19] Vanýsek, P., Ionic Conductivity and Diffusion at Infinite Dilution. In: CRC Handbook of Chemistry and Physics (D. R. Lide, editor), CRC Press, Boca Raton, FL, pp. 5-90, 1993. [ Links ] [20] Hines, A. L. and Maddox, R.N., Mass Transfer: Fundamentals and Applications, Prentice Hall, 1985. [ Links ] [21] HSC Chemistry for Windows version 5.0 Finland, 1999. http://www.outotec.com [ Links ] [22] Tecplot Inc., Tecplot 360, R 2012. http://www.tecplot.com/ Bellevue, WA 980062011. [ Links ]