Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
DYNA
versión impresa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.183 Medellín jan./feb. 2014
https://doi.org/10.15446/dyna.v81n183.36909
http://dx.doi.org/10.15446/dyna.v81n183.36909
TWO-DIMENSIONAL COMPUTATIONAL MODELING OF THE ELECTROKINETIC REMEDIATION OF A COPPER-CONTAMINATED SOIL PART II: SENSITIVITY ANALYSIS FOR A TRIANGULAR SOIL FIELD
MODELACION COMPUTACIONAL EN DOS DIMENSIONES DE LA REMEDIACION ELECTROCINETICA DE UN SUELO CONTAMINADO CON COBRE PARTE II: ANALISIS DE SENSIBILIDAD PARA UN CAMPO DE SUELO TRIANGULAR
VIRGILIO RUBIO-NIEBLAS
MSc., Universidad Autónoma de Baja California.Mexicali, México, virgilio62@gmail.com
MANUEL PEREZ-TELLO
PhD., Universidad de Sonora. Hermosillo, México, mperezt@iq.uson.mx
RICHARD A. JACOBS
PhD., Independent consultant. Houston, Texas, U.S.A, rajacobs@gmail.com
RONALDO HERRERA-URBINA
PhD., Universidad de Sonora. Hermosillo, México, rherrera@guaymas.uson.mx
Received for review January 26th, 2013, accepted October 8th, 2013, final version November, 12th, 2013
ABSTRACT: The computer model described in the first paper of this series was used to perform a sensitivity analysis for the electrokinetic remediation of a triangular, copper-contaminated soil field in which one anode and two cathodes are placed in the vicinity of the triangle vertices. The input variables included the initial concentration of copper in the soil, the electrical potential applied between electrodes, and the absence or presence of wash water. The output variables included the cleanup efficiency and the fraction of copper eliminated from the soil 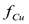 . Overall, the larger the electrical potential applied between electrodes, the shorter the time to achieve steady state. When no wash water is used, the cleanup efficiency may fluctuate over time before reaching steady state. When wash water is used, the final
. Overall, the larger the electrical potential applied between electrodes, the shorter the time to achieve steady state. When no wash water is used, the cleanup efficiency may fluctuate over time before reaching steady state. When wash water is used, the final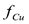 obtained depends on the initial concentration of copper in the soil.
obtained depends on the initial concentration of copper in the soil.
Key words: Two-dimensional, mathematical model, electrokinetics, sensitivity analysis
RESUMEN: El modelo computacional descrito en el primer artículo de esta serie se usó para realizar un análisis de sensibilidad del proceso de eliminación electrocinética de un campo de suelo triangular contaminado con cobre, en el cual se colocan un ánodo y dos cátodos en la proximidad de los vértices del triángulo. Las variables de entrada incluyeron la concentración inicial de cobre en el suelo, el potencial eléctrico aplicado entre los electrodos, y la ausencia o presencia de agua de lavado. Las variables de salida incluyeron la eficiencia de limpieza y la fracción de cobre eliminado del suelo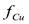 . En general, a mayor potencial eléctrico aplicado entre los electrodos, menor el tiempo requerido para alcanzar el estado estacionario. Cuando no se usa agua de lavado, la eficiencia de limpieza puede fluctuar en el tiempo antes de alcanzar el estado estacionario. Cuando se usa agua de lavado, el valor final de
. En general, a mayor potencial eléctrico aplicado entre los electrodos, menor el tiempo requerido para alcanzar el estado estacionario. Cuando no se usa agua de lavado, la eficiencia de limpieza puede fluctuar en el tiempo antes de alcanzar el estado estacionario. Cuando se usa agua de lavado, el valor final de 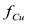 depende de la concentración inicial de cobre en el suelo.
depende de la concentración inicial de cobre en el suelo.
Palabras clave: Dos dimensiones, modelo matemático, electrocinética, análisis de sensibilidad
1. INTRODUCTION
Heavy metals in soils are important nutrients for plants in low concentrations, but at high concentrations they become toxic [1]. The contamination of soils by heavy metals is nowadays a major concern worldwide. In Part I of this series, a two-dimensional computational model for the electrokinetic remediation of a copper-contaminated soil was presented. The model is an adaptation of the original code developed by Jacobs and Probstein [2], and incorporates electromigration, electroosmosis, ordinary diffusion, and convection transport mechanisms. Based on a previous study reported by Fuerstenau and Palmer [3] on the distribution of copper species in aqueous solution, the chemical system was represented by 13 chemical species which participate in 8 chemical reactions. The computer model was validated by comparing their predictions with experimental data collected in a laboratory-scale system containing a soil collected at the city of Cananea, Mexico. A reasonable agreement between the predicted and the experimental values was obtained in terms of the spatial distributions of pH and copper concentration when the initial copper content in the soil was 100 and 600 mg/L . Once the computational model was verified, it can be used to analyze the main features of the electrokinetic remediation process. The potential applications of a two-dimensional formulation were pointed out by Jacobs [4], who conducted a computational study in which a number of electrode configurations was tested. Jacobs [4] found that electrode arrangements in which the anodes are surrounded by a number of cathodes provide greater efficiencies than those in which the cathodes are surrounded by the anodes. The simplest configuration obeying this criterion is a triangular arrangement in which one anode and two cathodes are placed in the vicinity of the triangle vertices. In a recent experimental study at laboratory scale, Almeira et al. [5] found that a triangular arrangement consisting of one anode and two cathodes forming three 60° angles between the lines connecting the electrodes was the most efficient in terms of both copper elimination and energy efficiency. Such experimental results [5] and further observations by Almeira et al. [6] and Peng et al. [7] experimentally confirmed the validity of the model predictions reported by Jacobs [4]. Based on the previous results shown in Part I of this series and the analysis of the relevant literature [4-7], the goal of this investigation was to test the effects of the main operating variables on the overall performance of the electrokinetic remediation for a large, triangular, two-dimensional soil field. For that purpose, a number of computer simulations were conducted with the mathematical model described in Part I of this series, as described below.
2. SIMULATION STRATEGY
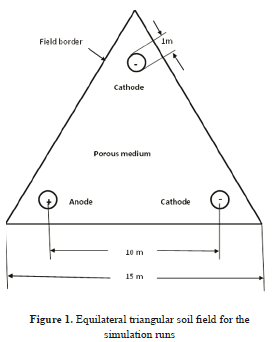
Figure 1 shows the system under study. An equilateral triangular soil field was assumed in which one anode and two cathodes are placed in the vicinity of the triangle vertices. The test variables included the initial concentration of copper in the soil pores (100 and 600 mg/L) and the electrical potential applied to the electrodes (500 and 1000 V).
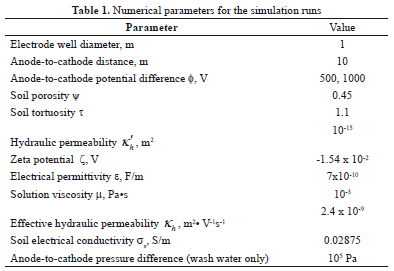
The specification of the latter values was based on the criteria reported in the literature [4], which suggests the application of 1 V per centimeter of separation between electrodes. Table 1 shows the numerical parameters used for the simulation runs, in which the properties of the Cananea soil reported in Part I of this series were assumed.
For every combination of the input variables, computer simulations were done for the two alternative cases in which pure wash water is either injected or not injected through the anode well. Because the mechanisms governing the motion of the chemical species in the soil are the same with and without water injection, the mathematical formulation can be used to predict the performance of the system under both types of operation strategies. For each case, a corresponding indicator of the process yield was defined as follows. In the simplest case, no wash water enters the soil, and thus the copper species are retained within the soil. At sufficiently long times, the copper is expected to concentrate within a localized area in the vicinity of the cathodes.
This soil will eventually be collected for further treatment or disposal. A feasible response variable for this case is the fraction of the total area in the arrangement which has been cleaned up to a given time t. In this study, this quantity is referred to as cleanup efficiency (CE); thus
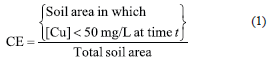
In this definition, the 50 mg/L limit appearing in the numerator was established based on local regulations. Although Equation 1 suggests that the CE may take on values in the range of zero to unity, in practical terms it may not reach the value of unity. This is because copper does not leave the system when no wash water is used. The calculation of the numerator in Equation 1 was accomplished within the framework of the Tecplot visualization software [8]. The procedure involves the calculation of the two-dimensional contours of copper concentration from the local concentration values within the computational domain, followed by the numerical calculation of the areas enclosed by those contours which comply the criterion shown in Equation 1.
An alternative way of operation consists of injecting pure wash water through the anode to maintain a continuous flow of the aqueous solution throughout the soil. This flow gradually takes the copper out of the system through the cathode wells. From a computational point of view, this mode of operation was simulated by setting a pressure difference between electrodes, as shown in Table I. In this case, an indicator of the process efficiency is the fraction of the initial copper in the soil which has been eliminated from the soil up to a given time t. This quantity is represented by the symbol 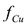 and was computed from the following expression:
and was computed from the following expression:
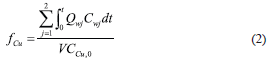
in which the numerator accounts for the amount of copper that left the soil through the cathode wells up to time t, and the denominator is the initial amount of copper in the soil field.
3. DISCUSSION OF RESULTS
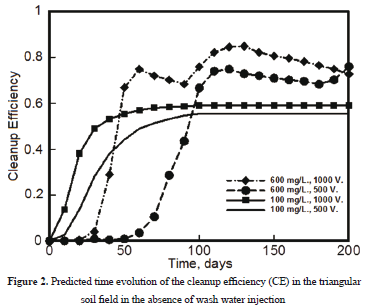
Figure 2 shows the predicted values of the CE in the absence of wash water in the triangular soil field. For all cases considered, the maximum CE values were predicted to be 0.55 and 0.59 when the electrical potential difference was set to 500 and 1000 V, respectively. Overall, the effect of the electrical potential difference is mostly on the process kinetics. The larger the electrical potential difference, the faster the initial response of the CE, and thus the shorter the time to achieve steady state. This behavior may be explained in terms of the electroosmosis and electromigration transport mechanisms, which are both dependent upon the electrical potential gradient 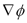 according to the following expressions [2]
according to the following expressions [2]
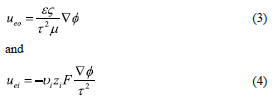
where 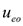 and
and 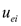 are the electroosmotic and electromigration velocities, respectively, and other symbols are defined in the nomenclature. It is noted that
are the electroosmotic and electromigration velocities, respectively, and other symbols are defined in the nomenclature. It is noted that 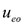 represents the velocity of the aqueous solution adjacent to the pore walls; thus, it affects all species in solution at a given location. On the other hand, the electromigration velocity
represents the velocity of the aqueous solution adjacent to the pore walls; thus, it affects all species in solution at a given location. On the other hand, the electromigration velocity 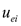 is only attributed to those chemical species in solution carrying an electrical charge
is only attributed to those chemical species in solution carrying an electrical charge 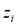
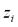 . From a macroscopic standpoint, any change in the electrical potential difference between the electrodes will affect the local electrical potential gradient
. From a macroscopic standpoint, any change in the electrical potential difference between the electrodes will affect the local electrical potential gradient 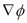 , and thus the kinetics of the overall process described by Equations (3) and (4). On the other hand, it is of interest to note the values of CE at long times, i.e., once steady state was achieved. Such CE values increased as the initial concentration of copper in the soil also increased. Therefore, as far as the cleanup efficiency is concerned, highly contaminated soils are expected to be cleaned up more efficiently than moderately contaminated soils. A complementary response variable is the time to reach steady state conditions. This quantity was defined as the time at which the cleanup efficiency CE varied by less than 0.01 percent from one time step to the next. In this study, this quantity is represented by the symbol:
, and thus the kinetics of the overall process described by Equations (3) and (4). On the other hand, it is of interest to note the values of CE at long times, i.e., once steady state was achieved. Such CE values increased as the initial concentration of copper in the soil also increased. Therefore, as far as the cleanup efficiency is concerned, highly contaminated soils are expected to be cleaned up more efficiently than moderately contaminated soils. A complementary response variable is the time to reach steady state conditions. This quantity was defined as the time at which the cleanup efficiency CE varied by less than 0.01 percent from one time step to the next. In this study, this quantity is represented by the symbol: 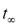 . Figure 2 shows that
. Figure 2 shows that 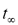 was in the range of 90-100 days when the initial copper content was 100 mg/L, whereas it was longer than 200 days when the initial copper content was 600 mg/L.
was in the range of 90-100 days when the initial copper content was 100 mg/L, whereas it was longer than 200 days when the initial copper content was 600 mg/L.
The initial copper concentration in the soil also affected the kinetics of the CE. Thus, when it was set to 100 mg/L, the CE increased with time up to an asymptotic value which depended upon the electrical potential difference applied to the soil. In contrast, when the initial copper concentration was set to 600 mg/L, the CE values showed significant fluctuations with time before reaching steady state conditions.
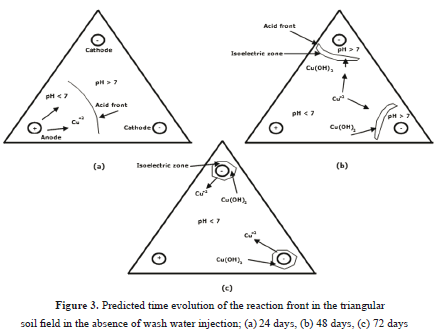
Computer visualizations of the concentration field in this time period provided an explanation for this behavior. A simplified diagram of such calculations is shown in Figure 3, which shows that the local values of pH are strongly related to the distribution of the chemical species throughout the soil. At t=24 days (Figure 3a) the H+ ions produced at the anode moved to the cathodes, thus creating an acid zone.
At the same time, the OH- ions produced at the surface of the cathodes moved toward the anode, thus forming a basic zone. The locations at which both species meet in the soil is characterized by a sudden change in pH values as a result of the neutralization reaction: 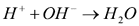 . The locations at which this reaction occurs is referred to in this paper as the reaction front, and depends upon the mobilities of both H+ and OH- ions [2], as well as the concentrations of the anionic and cationic copper species in solution.
. The locations at which this reaction occurs is referred to in this paper as the reaction front, and depends upon the mobilities of both H+ and OH- ions [2], as well as the concentrations of the anionic and cationic copper species in solution.
At these locations, both solid and aqueous Cu(OH)2 are formed. Because aqueous Cu(OH)2 carries no electrical charge, it causes the electrical conductivity of the aqueous phase to decrease substantially, thus forming an isoelectric zone. The coexistence of both solid and aqueous Cu(OH)2 occurs in the range of: 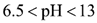 , whereas in the range of
, whereas in the range of 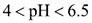 only the aqueous form is present [9]. As the acid front advanced at t=48 days (Figure 3b), the reaction front and the isoelectric zone became narrow. This is because the H+ ions are continuously produced at the anode surface and react to form other cations such as Cu(OH)2+ and Cu(OH)2+2, which also moved toward the cathodes. On the surface of the cathodes, the OH- ions are also formed and react to produce other anions such as HCuO2- and CuO2- which move toward the anode. This process repeats over time (t=72 days, Figure 3c) until steady state is achieved. The fluctuating motion of the copper ions throughout the soil thus explains the fluctuating behavior of CE in Figure 2.
only the aqueous form is present [9]. As the acid front advanced at t=48 days (Figure 3b), the reaction front and the isoelectric zone became narrow. This is because the H+ ions are continuously produced at the anode surface and react to form other cations such as Cu(OH)2+ and Cu(OH)2+2, which also moved toward the cathodes. On the surface of the cathodes, the OH- ions are also formed and react to produce other anions such as HCuO2- and CuO2- which move toward the anode. This process repeats over time (t=72 days, Figure 3c) until steady state is achieved. The fluctuating motion of the copper ions throughout the soil thus explains the fluctuating behavior of CE in Figure 2.
It is noted that the present formulation assumes that the transport of the chemical species occurs in the aqueous phase only, i.e., the solid phase is assumed to be motionless. It is also assumed that the soil is isotropic and its properties do not vary with time. Therefore, any change in the rate of transport of the chemical species may be attributed to changes occurring in the aqueous phase only. The incorporation of anisotropies, time-dependent soil properties, and other phenomena such as electrophoresis was beyond the scope of this investigation.
The results shown in Figure 3 qualitatively agree with the experimental observations by Almeira et al. [5,6] and Peng et al. [7] regarding the formation of three distinctive areas within the soil; namely, and acid region, a basic region, and a narrow area with low electrical conductivity. Overall, the results shown in Figures 2 and 3 indicate that the behavior of this type of a system in the absence of wash water injection is complex, and cannot be generalized. Detailed simulations for specific field dimensions, soil characteristics, and operating conditions must be done in order to analyze the process performance prior to its optimization.
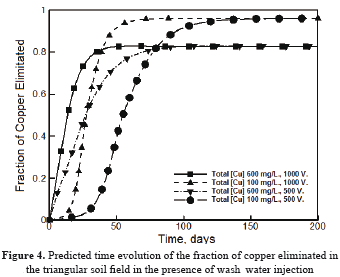
Figure 4 shows the predicted behavior of the fraction of copper eliminated 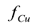 computed from Equation (2) when pure wash water was used. Under the test conditions, the maximum values of
computed from Equation (2) when pure wash water was used. Under the test conditions, the maximum values of 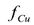 were predicted to be in the range of 0.8-0.95. At long times, all the curves approach an asymptotic value of
were predicted to be in the range of 0.8-0.95. At long times, all the curves approach an asymptotic value of 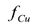 which is dependent upon the initial concentration of copper in the soil, and is independent of the electrical potential difference applied to the electrodes. The asymptotic values of
which is dependent upon the initial concentration of copper in the soil, and is independent of the electrical potential difference applied to the electrodes. The asymptotic values of 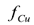 at sufficiently long times decreased as the initial copper concentration increased. Therefore, in terms of the fraction of copper eliminated from the soil
at sufficiently long times decreased as the initial copper concentration increased. Therefore, in terms of the fraction of copper eliminated from the soil 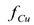 , the more dilute the copper in the original soil, the more efficient the electrokinetic remediation process.
, the more dilute the copper in the original soil, the more efficient the electrokinetic remediation process.
This behavior contrasts with that observed for the cleanup efficiency in the absence of wash water injection shown in Figure 2. The time to reach steady state conditions throughout the soil was defined as the time at which the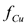 value varied less than 0.01 percent from one time step to the next. Figure 4 shows that this quantity is strongly dependent upon the electrical potential difference applied to the electrodes. Thus, the higher the electrical potential, the shorter the time to reach steady state conditions and vice versa. As was discussed previously, this is attributed to the electroosmotic and electromigration velocities, which are dependent upon the electrical potential gradient according to Equations 2 and 3, respectively. The behavior of the system under the presence of wash water injection shows a simpler behavior than that observed when no wash water is injected to the soil. However, further studies are necessary to clarify whether the trends observed in this study for a triangular soil field can be generalized to other electrode arrangements.
value varied less than 0.01 percent from one time step to the next. Figure 4 shows that this quantity is strongly dependent upon the electrical potential difference applied to the electrodes. Thus, the higher the electrical potential, the shorter the time to reach steady state conditions and vice versa. As was discussed previously, this is attributed to the electroosmotic and electromigration velocities, which are dependent upon the electrical potential gradient according to Equations 2 and 3, respectively. The behavior of the system under the presence of wash water injection shows a simpler behavior than that observed when no wash water is injected to the soil. However, further studies are necessary to clarify whether the trends observed in this study for a triangular soil field can be generalized to other electrode arrangements.
4. CONCLUDING REMARKS
The computer simulation of the electrokinetic remediation of a copper-contaminated soil field with a triangular arrangement of electrodes was performed. The results indicated that the electrical potential difference applied between electrodes mostly affects the process kinetics due to the electroosmotic and electromigration velocities.
Also, the initial copper content in the soil determines the final process efficiency. When no wash water is injected to the soil, the final cleanup efficiency increased as the initial content of copper in the soil was increased, and it may fluctuate over time before reaching steady state conditions. Such fluctuations may be explained in terms of the chemical reactions occurring at both sides of the acid front moving throughout the soil during the operation.
On the other hand, when wash water is injected, the final fraction of copper eliminated from the soil depends upon the initial concentration of copper in the soil only. The results obtained in this study illustrate the potential use of the present formulation for the further optimization of this process.
NOMENCLATURE

REFERENCES
[1] Hernán, S.L., González, L.M. y Espinosa, A., Modelación de elementos traza en el horizonte de suelos, plancha 170 (Vélez, Departamentos de Santander y Boyacá), (in spanish). Dyna, 156, pp. 157-164, 2008. [ Links ] [2] Jacobs R.A., Probstein, R.F. Two-dimensional modeling of electroremediation. AIChE J., 42(6), pp. 1685-1696, 1996. [ Links ] [3] Fuerstenau, M.C. and Palmer, B.R., Anionic flotation of oxides and silicates. In: Flotation - A. M. Gaudin Memorial Volume-Volume 1, (M.C. Fuerstenau, Editor), Chapter 7, American Institute of Mining, Metallurgical, and Petroleum Engineers Inc., New York, pp. 148-196, 1976 [ Links ] [4] Jacobs, A. R., Removal of contaminant material from a soil site. US Patent No. 5415744, 1995. [ Links ] [5] Almeira, J., Peng, Ch. and Wang, Z., Effect of different electrode configurations on the migration of copper ions during the electrokinetic remediation process. Asia-Pac. J. Chem. Eng., 4, pp. 581-585, 2009. [ Links ] [6] Almeira, J., Peng, Ch. and Li, P., Effect of electrode configuration on the distribution of Cu during electrokinetic soil remediation. J. Kor. Soc. Urb. Env., 10(2), 2010. [ Links ] [7] Peng, Ch., Almeira, J. and Gu, Q., Effect of electrode configuration on pH distribution and heavy metal ions migration during soil electrokinetic remediation. Env. Earth Sci., 69, pp. 257-265, 2013. [ Links ] [8] Tecplot Inc., Tecplot 360, R 2012. http://www.tecplot.com/ Bellevue, WA 980062011 [ Links ] [9] Jacobs, R.A., Sengun, M.Z., Hicks, R.E. and Probstein, R.F., Model and experiments on soil remediation by electric fields. J. Env. Sci. Health Part A-Environmental Science and Engineering & Toxic and Hazardous Substance Control, 29(9), pp. 1933-1955, 1994. [ Links ]