Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.185 Medellín May/June 2014
https://doi.org/10.15446/dyna.v81n185.33409
http://dx.doi.org/10.15446/dyna.v81n185.33409
Phase transformations in air plasma-sprayed yttria-stabilized zirconia thermal barrier coatings
Transformaciones de fase en recubrimientos de barrera térmica de zirconia estabilizada con yttria depositados mediante aspersión por plasma atmosférico
Julián D. Osorio a, Adrián Lopera-Valle b, Alejandro Toro c & Juan P. Hernández-Ortiz d
a Materials and Minerals Department, National University of Colombia, Medellín, Colombia, jdosorio@unal.edu.co
b Mechanical Engineering, National University of Colombia, Medellín, Colombia, adloperav@unal.edu.co
c Tribology and Surfaces Group, National University of Colombia, Medellín, Colombia, aotoro@unal.edu.co
d Materials and Minerals Department, National University of Colombia, Medellín, Colombia, jphernandezo@unal.edu.co
Received: September 24th, 2012. Received in revised form: January 23th, 2014. Accepted: January 27th, 2014
Abstract
Phase transformations in air plasma-sprayed thermal barrier coatings composed of ZrO2 – 8 wt.% Y2O3 (zirconia - 8 wt.% yttria) are studied using X-Ray diffraction and Rietveld refinement measurements. Samples of TBC deposited onto Inconel 625 substrate were fabricated and heat treated at two different conditions: exposition to 1100°C up to 1000 hours and exposition to temperatures between 700°C and 1100°C during 50 hours. According to Rietveld refinement measurements, the content of the cubic phase in the top coat increases with time and temperature; it starts at 7.3 wt.% and reaches 15.7 wt.% after 1000 hours at 1100°C. The presence of a cubic phase in high amounts is undesirable due its lower mechanical properties compared with the tetragonal phase. After 800 hours of exposure to high temperature, the amount of Y2O3 in the tetragonal phase reduces to 6.6 wt.% and a fraction of this phase transforms to a monoclinic structure during cooling. The monoclinic phase reached 18.0 wt.% after 1000 hours. This phase is also undesirable, not only due to its higher thermal conductivity, but also because the tetragonal-to-monoclinic transformation implies a volume change of circa 5%, which favors crack formation and propagation and compromises the coating integrity.
Keywords: Thermal Barrier Coating (TBC); Heat Treatment; Phase Transformation; Rietveld Analysis.
Resumen
En este trabajo, las transformaciones de fase en Recubrimientos de Barrera Térmica (TBC) constituidos por ZrO2 – 8 wt.% Y2O3 (zirconia - 8 wt.% ytrria) fueron estudiados a través de Difracción de Rayos X (XRD) y refinamiento Rietveld. Las muestras de TBC fueron depositadas mediante aspersión por plasma atmosférico sobre un sustrato tipo Inconel 625 y fueron tratadas térmicamente con dos condiciones diferentes: en la primera se utilizó una temperatura de 1100°C con tiempos de exposición entre 1 hora y 1000 horas; en la segunda las muestras fueron sometidas a temperaturas entre 700°C y 1100° durante 50 horas. De acuerdo a los resultados obtenidos mediante refinamiento Rietveld el contenido de fase cúbica en el recubrimiento (TC) se incrementa con el tiempo y la temperatura, desde 7.3 wt.% hasta 15.7 wt.% después de 1000 horas a 1100°C. La fase cúbica en grandes cantidades es indeseable debido a que presenta inferiores propiedades mecánicas cuando se compara con la fase tetragonal. Después de 800 horas de exposición a alta temperatura, el contenido de Y2O3 en la fase tetragonal se reduce hasta 6.6 wt.% y una fracción de la fase tetragonal transforma a monoclínica durante el enfriamiento. La fase monoclínica alcanza 18.0 wt.% después de 1000 horas. Esta fase es también indeseable porque además de tener una mayor conductividad térmica, la transformación de tetragonal a monoclínica viene acompañada de un cambio volumétrico de alrededor de 5% que promueve la formación y propagación de grietas, las cuales comprometen la integridad del recubrimiento.
Palabras clave: Recubrimiento de Barrera Térmica (TBC); Tratamiento Térmico; Transformaciones de fase; Refinamiento Rietveld.
1. Introduction
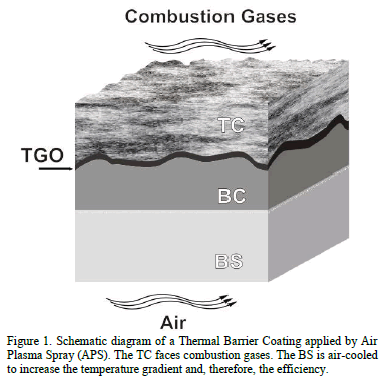
Thermal barrier coatings (TBCs) are multilayered systems widely used in gas turbines to increase efficiency and durability [1-4]. These coatings consist of three layers deposited onto a Base Substrate (BS): the Top Coat (TC), the Bond Coat (BC) and the Thermally Grown Oxide (TGO) layer (see Fig. 1). Base substrates are usually Ni-based superalloys that offer good mechanical strength and excellent corrosion, oxidation and erosion resistances at high temperatures [5-7]. They contain significant amounts of alloying elements such as Cr, Mo, Al, Ti, Fe and C, which favor intermetallic compounds precipitation [8,9]. Two types of BCs are commonly used: the Platinum- modified Nickel
Aluminide (PtNiAl) and MCrAlY alloys, where M refers to one or more of the elements Co, Ni and Fe. The BC is a metallic layer that initially provides adherence between the TC and the substrate [10,11]. During operation at high temperatures, aluminum diffuses from the BC and reacts to form a barrier layer, known as the TGO. Once the TGO is formed, the BC serves as the anchoring layer between the TC and the TGO. The TGO provides the barrier to oxygen diffusion to avoid substrate oxidation. However, many of the failure mechanisms in TBCs are related to the TGO formation and growth [12-16].
The TC is usually composed by Yttria (Y2O3) stabilized Zirconia (ZrO2), and serves as the main defense mechanism of gas turbines against erosion and corrosion. The TC has low thermal conductivity that reduces the temperature of the bond coat up to 500°C with a thickness of some hundred microns. It must be stabilized in order to maintain its tetragonal structure at room temperature and also to keep thermal properties constant (conductivity and thermal expansion coefficient) in the range of working temperatures. To accomplish this stabilization, some elements such as Hafnium (Hf) and Yttrium (Y), are commonly added [17-20]. The Yttrium (Y+3) and Hf (Hf+4) ions replace the zirconium (Zr+4) ions in the lattice cell inducing changes in the crystal structure. These changes stabilize the tetragonal phase and decrease the thermal conductivity. In the first case, the Y+3 ions produce oxygen vacancies in the lattice [18], while the Hf+4 ions, which are chemically similar and have comparable ionic radius to Zr+4 ions, are almost twice as massive and generate a lattice disorder. In both cases, thermal conductivity is reduced due to an un-harmonic scatter of the charge carriers in the ceramics at high temperature, i.e. un-harmonic phonon scatter phenomenon [19,21].
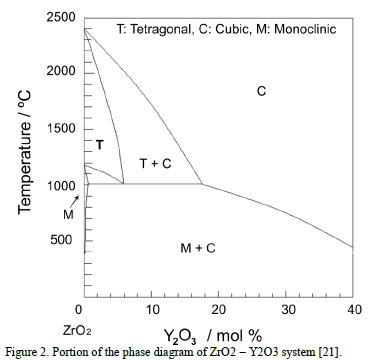
Zirconia without stabilizers can exist in three different phases [18,22]: cubic, tetragonal and monoclinic, see the phase diagram [23] in Fig. 2. The tetragonal-to- monoclinic
transformation is undesirable because it is accompanied by a volumetric change of circa 5%, which causes a detrimental effect to the TBC due to crack nucleation and propagation [24,25]. In addition, the tetragonal phase presents excellent mechanical and thermal properties compared with those of the monoclinic phase [2,19,26,27]. The tetragonal-to-monoclinic transformation is avoided with the addition of 6 to 8 wt.% of Yttria [18].
Two different processes are currently used to deposit Yttria stabilized Zirconia: Electron Beam-Physical Vapor Deposition (EB-PVD) and Air Plasma Spray (APS). In both cases, the rapid cooling results in a metastable tetragonal phase (t') rather than a stable tetragonal [28,29]. According to some studies, during exposure to high temperature and during cycling operations, Yttrium (Y) diffuses from the t' phase to stabilize the cubic phase [30,31]. Consequently, a monoclinic phase appears from the Y-depleted tetragonal phase during cooling.
The presence of both, cubic phase in high amounts and monoclinic phase is undesirable due to their lower mechanical properties compared with the tetragonal phase. Also, the volumetric changes associated with the phase transformations favor crack generation and propagation which compromise the coating integrity. Therefore, understanding these transformations is essential to find alternatives to improve the TBC's lifetime.
In this work, phase transformations in an APS-deposited TC under two different sets of heat treatments are studied using X-Ray Diffraction (XRD) and semi-quantitative Rietveld refinement. The paper is organized as follows: in Section 2, the experimental procedure, methods and materials are presented. Section 3 describes a summary of the results, where the effects of exposure time at 1100°C are analyzed first and then the phase dynamics for different exposure temperatures during 50 hours is discussed. The most important conclusions are summarized at the end.
2. Experimental procedure
The TBC samples are composed of a ZrO2 – 8 wt.% Y2O3 TC applied by APS onto a NiCrCoAlY BC, both layers having thicknesses around 300 µm. The BC layer was deposited onto a nickel-base substrate, namely Inconel 625. The dimensions of the TBC samples were 10 cm x 10 cm, extracted from plates of 30 cm x 30 cm. The samples were cut with a precision saw operating at 4000 RPM. Thereafter, some samples were heated at a rate of 18 °C/min and maintained at 1100°C between 1 and 1000 hours, while other samples were thermally treated for 50 hours at 700°C, 800°C, 900°C and 1000°C; In all cases, the samples were cooled in air.
Sample preparation included grinding with No. 400 and No. 600 emery papers for 5 minutes, followed by polishing by cloths with abrasive diamond suspensions containing particles with 12 µm, 6 µm, 3 µm and 1 µm in average size. The polishing time for the first three suspensions was 15 minutes, while 60 minutes were required for the 1 µm suspension. A complete characterization of this material with similar heat treatment conditions has been reported in previous works [32,33].
The phase characterization was carried out in a Panalytical X Pert Pro MPD X-Ray Diffractometer with a CuKa (l = 1.5406 Å) radiation gun within a 20° < 2q; <100° range and step of 0.013°/seg. Rietveld semi-quantitative measurements were made to account the phase changes at different temperature conditions. To ensure reliability of the results, two replicas of each sample were also measured. The software used to perform the Rietveld refinements was X'Pert High Score Plus Version 2.2a by PANalytical B.V. It is well known that the tetragonal lattice parameters change depend on the Y2O3 content. Therefore, Rietveld measurements were also performed to determine the lattice tetragonal parameters a and c, in order to determine the amount of Y2O3 in this phase. The amount of Y2O3 in the tetragonal phase, for each heat treatment condition, was determined using the following relation [22,34]:
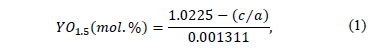
where a and c are the lattice tetragonal parameters in nanometers. This expression was derived by H.G. Scott in 1975 [22], based on the change of lattice parameters of Yttria Stabilized Zirconia powders with different YO1.5 content; it was corrected empirically by Ilavsky and Stalick [34] to improve the fit throughout the annealing process to use it in a wide range of samples [35].
3. Results and analysis
In the as-sprayed condition, the TC was a mixture of 92.7 wt.% tetragonal and 7.3 wt.% cubic phases. In this condition, the Y2O3 content in the tetragonal phase was 7.53 wt.% (~7.53 mol% YO1.5) which is within the recommended range for which the tetragonal-to-monoclinic transformation
is prevented after cooling [18]. Some researchers have reported a TC consisting exclusively of the tetragonal phase [31] and others have found considerable amounts of the monoclinic phase in the as-sprayed condition [34,36]. The differences in the initial compositions and microstructure depend, not only on the stabilizers' content, but also on the feedstock [34] and the presence of unmelted or partially melted particles [36].
3.1. Effect of exposure time at 1100°C
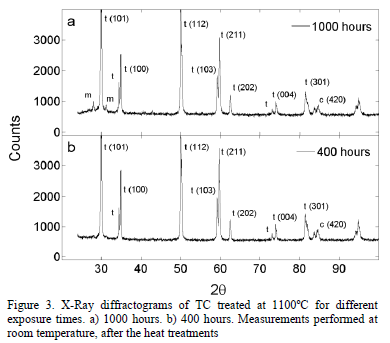
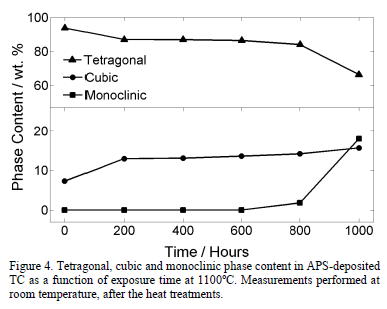
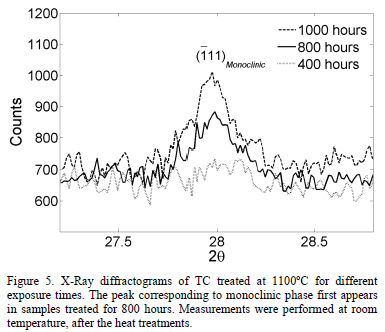
Fig. 3 presents two x-ray diffractograms for the samples thermally treated during 1000 hours and 400 hours at 1100°C, respectively. It can be observed that the monoclinic phase appears in the sample treated for 1000 hours. The evolution of the TC phases after the exposure to 1100°C, measured through Rietveld refinement, is shown in Fig. 4. The tetragonal phase decreases from 97.3 wt.% to 66.3 wt.% after 1000 hours, while the cubic phase increases from 7.3 wt.% to 15.7 wt.%. At 800 hours, the monoclinic phase rapidly starts to form and, after 1000 hours, it reaches 18.0 wt.%. This is observed both in
Fig. 4 and in the X-Ray diffractogram in Fig. 5. The uncertainty in the phase content was determined using the standard deviation. The uncertainty value was around 0.38 wt.% with a maximum of 0.43 wt.%
Fig. 4 also shows that the cubic phase forms in the first 200 hours at the expense of the tetragonal phase. Then, the cubic phase growth proceeds at a slower rate. After 800 hours, the monoclinic phase increases quickly, overpassing the cubic content before 1000 hours.
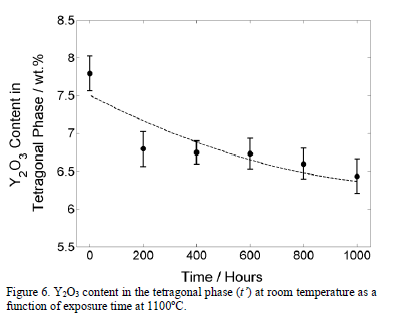
Fig. 6 shows how the Yttrium content of the metastable t' phase at room temperature reduces with exposure time at 1100°C. It is known that the t' phase decomposes into a mixture of stable tetragonal and cubic phases [37] as a consequence of Yttrium diffusion. However, for crystallographic purposes, both tetragonal structures can be analyzed as the same tetragonal polymorph in the zirconia solid solution [28,38].
As can be observed in Fig. 6, the Y2O3 content constantly decreases in the tetragonal (t') phase with exposure time. After 800 hours, the Y2O3 content in t' phase decreases to 6.60 wt.%. Then, the Y-depleted tetragonal phase transforms to a monoclinic phase during cooling and the monoclinic phase becomes more stable, favored by the Yttrium reduction in the tetragonal phase. Another factor which promotes the tetragonal-to-monoclinic phase transformation is the grain size [36,39,40]. From thermodynamic formulations, some researchers [39] have found that the surface energy of the tetragonal phase is lower than that of the monoclinic phase for a grain size smaller than 200 nm. Then, for a grain size smaller than 200 nm, the tetragonal phase is more stable. In addition, greater grain sizes favor the diffusion rate through the grain boundaries [36]. Therefore, it can be concluded that the Yttrium diffusion from the tetragonal phase increases and the amount of monoclinic phase formed from the Yttrium-depleted tetragonal phase increases.
3.2. Effect of the treatment temperature for fixed exposition time
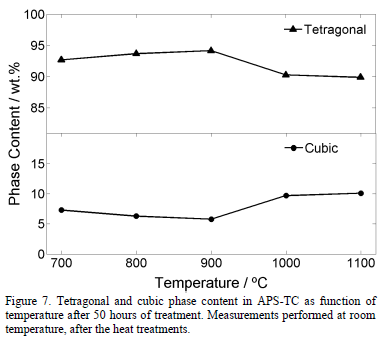
The results of Rietveld measurements performed in the samples treated at different temperatures for 50 hours are presented in Fig. 7. The cubic phase increases slightly (around 3 wt.%) from 700°C to 1100°C. The uncertainty was around 0.41 wt.% with a maximum value of 0.44 wt.%. No monoclinic phase was detected in any treatments. Therefore, it can be said that the tetragonal phase decreases in the same proportion as the cubic phase increases. On the other hand, the cubic content increases with both temperature and exposure time. This behavior is in agreement with the results found in the literature in which the cubic content after 100 hours at 1200°C is around 19.0 wt.% and it reaches more than 40.0 wt.% for heat treatments at 1400°C after 100 hours [31].
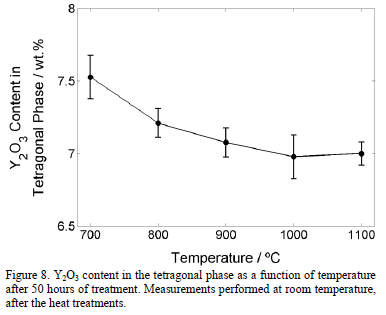
The effect of the temperature in the Y2O3 content in the tetragonal phase is shown in Fig. 8. A slight reduction from 7.5 wt.% to around 7.0 wt.% is observed. On the other hand, the slight increment in cubic content is probably caused by the diffusion of the Yttrium from the tetragonal (t') phase to stabilize the cubic phase. According to the results presented in Fig. 6, the monoclinic phase appears when the Y2O3 content reduces to 6.6 wt.% or below. Therefore, it is not expected that the monoclinic phase forms after 50 hours at any temperature equal to or below 1100°C. The generality of the value of 6.6 wt.% of Y2O3 in the tetragonal phase that was found in this work, at which the tetragonal phase destabilizes to transform in monoclinic phase during cooling requires additional research. Other factors, such as grain size and stresses, can favor the monoclinic stabilization.
4. Conclusions
The phase transformations in APS-deposited TCs composed of ZrO2 – 8 wt.% Y2O3 under different heat treatment conditions were studied through XRD and Rietveld semi-quantitative measurements. The tetragonal structure (t') generated from the APS deposition process became unstable at high temperatures. The increase in temperature and exposure time favored Yttrium diffusion from the tetragonal phase and promoted formation of the cubic phase. The amount of such a cubic phase increased from 7.3 wt.% at room temperature to 15.7 wt.% after 1000 hours at 1100°C. After 800 hours at 1100°C, the monoclinic phase started to form and the Y2O3 content in the tetragonal phase reduced to values below 6.6 wt.%; the amount of the monoclinic phase increased rapidly and reached 18.0 wt.% after 1000 hours at this temperature.
Acknowledgements
The authors thank COLCIENCIAS and Empresas Públicas de Medellín (EPM) for funding this investigation through the project No. 111845421942. The authors are also grateful to the Materials Characterization Laboratory at the National University of Colombia at Medellín, for providing the characterization instruments.
References
[1] Boyce, P. M., Gas Turbine Engineering Handbook, Gulf Professional Publishing, Second Edition, 2002. [ Links ]
[2] Padture ,N. P., et al. Thermal Barrier Coatings for Gas-Turbine Engine Applications, Science 296, 280, 2002. [ Links ]
[3] Trice, R. W., Su, Y. J., Mawdsley, J. R. and Faber, K. T., Effect of heat treatment on phase stability, microstructure, and thermal conductivity of plasma-sprayed YSZ, Journal Of Materials Science 37, pp. 2359-2365, 2002. [ Links ]
[4] Sivakumar, R. and Mordike, B. L. High temperature coatings for gas turbine blades: a review, Surface and coatings technology 37, pp. 139 -160, 1989. [ Links ]
[5] Davis, J. R., Heat Resistant Materials (ASM Specialty Handbook), ASM International, 1997. [ Links ]
[6] Rai, S. K., Kumar, A., Shankar, V., Jayakumar, T. et al. Characterization of microstructures in Inconel 625 using X-ray diffraction peak broadening and lattice parameter measurements, Scripta Materialia 51, pp. 59–63, 2004. [ Links ]
[7] González, A., López, E., Tamayo, A., Restrepo, E. and Hernández, F., Microstructure and Phases Analyses of Zirconia-Alumina (ZrO2 - Al2O3) Coatings Produced By Thermal Spray, DYNA 77, no. 162, pp. 151-160, 2010. [ Links ]
[8] Reed, R. C., The Superalloys: Fundamentals and Applications, Cambridge University Press, 2006. [ Links ]
[9] Zhao, J. C., Larsen, M. and Ravikumar, V., Phase precipitation and time–temperature transformationdiagram of Hastelloy X, Materials Science and Engineering A293, pp. 112– 119, 2000. [ Links ]
[10] Nicoll, A. R. and Wahl, G., The effect of alloying additions on M-Cr-Al-Y Systems: an experimental study, Thin Solid Films, 95, pp. 21-34, 1982. [ Links ]
[11] Richard, C. S., Béanger, G., Lu J. and Flavenot, J. F., The influences of heat treatments and interdiffusion on the adhesion of plasma-sprayed NiCrAlY coatings, Surface and Coatings Technology 82, pp. 99-109, 1996. [ Links ]
[12] Spitsberg, I.T., Mumm, D.R. and Evans, A. G., On the failure mechanisms of thermal barrier coatings with diffusion aluminide bond coatings, Materials Science and Engineering A 394, pp. 176–191, 2005. [ Links ]
[13] Seo, D. and Ogawa, K., et al. Influence of high-temperature creep stress on growth of thermally grown oxide in thermal barrier coatings, Surface and Coatings Technology 203, pp. 1979–1983, 2009. [ Links ]
[14] Nychka, J. A., Xu, T., Clarke, D. R. and Evans, A. G., The stresses and distortions caused by formation of a thermally grown alumina: comparison between measurements and simulations, Acta Materialia 52, pp. 2561–2568, 2004. [ Links ]
[15] Osorio, J. D., Giraldo, J., Hernández, J. C., Toro, A. and Hernández-Ortiz, J. P., Diffusion–Reaction of Aluminum and Oxygen in Thermally Grown Al2O3 Oxide Layers, Heat and Mass Transfer 50, 483-492, 2014. [ Links ]
[16] Tolpygo, V. K., Clarke, D. R. Surface Rumpling of a (Ni, Pt) Al Bond Coat Induced by Cyclic Oxidation, Acta materialia 48, 3283-3293, 2000. [ Links ]
[17] Clarke, D R., Materials selection guidelines for low thermal conductivity thermal barrier coatings, Surface and Coatings Technology 163 –164, 67–74, 2003. [ Links ]
[18] Clarke, D. R., Levi, C. G., Materials design for the next generation thermal barrier coatings, Annu. Rev. Mater. Res. 33, pp. 383-417, 2003. [ Links ]
[19] Winter, M. R. and Clarke, D. R., Oxide Materials with low Thermal Conductivity, Journal of the American Ceramic Society, 90, pp. 533–540, 2007. [ Links ]
[20] Zhu, D. and Miller, R. A., Sintering and creep behavior of plasma-sprayed zirconia- and hafnia based thermal barrier coatings, Surface and Coatings Technology 108–109, pp. 114-120, 1998. [ Links ]
[21] Niranatlumpong, P., Ponton, C. B. and Evans, H. E., The Failure of Protective Oxides on Plasma-Sprayed NiCrAlY Overlay Coatings, Oxidation of Metals, Vol. 53, no. 3/4, 2000. [ Links ]
[22] Scott, H. G., Phase relationships In Zirconia-Yttria System Journal of Material Science 10, pp. 1527-1535, 1975. [ Links ]
[23] Fabrichnaya, O., Wang, C., Zinkevich, M., Levi, C. G. and Aldinger, F., Phase Equilibria and Thermodynamic Properties of the ZrO2-GdO1.5-YO1.5 System, Journal of Phase Equilibria 26 [6] pp. 591–604, 2005. [ Links ]
[24] VanValzah, J. R., Eaton, H. E. Cooling rate effects on the tetragonal to monoclinic phase transformation in aged plasma-sprayed yttria partially stabilized zirconia, Surface and Coatings Technology, 46, pp. 289-300, 1991. [ Links ]
[25] Xie, L., Jordan, E. H., Padture, N. P. and Gell, M., Phase and microstructural stability of solution precursor plasma sprayed thermal barrier coatings, Materials Science and Engineering A 381, pp. 189–195, 2004. [ Links ]
[26] Osorio, J. D., Maya, D., Barrios, A. C., Lopera, A., Jiménez, F., Meza, J. M., Hernández-Ortiz, J. P. and Toro, A., Correlations Between Microstructure and Mechanical Properties of Air Plasma-Sprayed Thermal Barrier Coatings Exposed to a High Temperature, Journal of the American Ceramic Society 96 [12], pp. 3901-3907, 2013. [ Links ]
[27] Busso, E. P., Qian, Z. Q., Taylor, M. P. and Evans, H. E., The influence of bond coat and topcoat mechanical properties on stress development in thermal barrier coating systems, Acta Materialia 57, pp. 2349–2361, 2009. [ Links ]
[28] Tsipas, S. A., Effect of dopants on the phase stability of zirconia-based plasma sprayed thermal barrier coatings, Journal of the European Ceramic Society 30, pp. 61–72, 2010. [ Links ]
[29] Ilavsky, J., Stalick, J. K. and Wallace, J., Thermal Spray Yttria-Stabilized Zirconia Phase Changes during Annealing, Journal of Thermal Spray Technology Volume 10(3), 497, 2001. [ Links ]
[30] Trice, R. W., Jennifer, Y., Mawdsley, J. R., Faber, K. T., Arellano-lopez R., Wang H. and Porter, W. D., Effect of heat treatment on phase stability, microstructure, and thermal conductivity of plasma-sprayed YSZ, Journal of Materials Science 37, pp. 2359 – 2365. 2002. [ Links ]
[31] Schulz, U., Phase Transformation in EB-PVD Yttria Partially Stabilized Zirconia Thermal Barrier Coatings during Annealing, Journal of the American Ceramic Society 83 [4], 904–10, 2000. [ Links ]
[32] Osorio, J. D., Hernández-Ortiz, J. P. and Toro, A., Microstructure Characterization of Thermal Barrier Coating Systems After Controlled Exposure to a High Temperature, Ceramics International 40, pp. 4663-4671, 2014. [ Links ]
[33] Osorio, J. D., Toro, A. and Hernández-Ortiz, J. P., Thermal Barrier Coatings for Gas Turbine Applications: Failure Mechanisms and Key Microstructural Features, DYNA 79, no. 176, pp 149-158, 2012. [ Links ]
[34] Ilavsky, J. and Stalick, J. K., Phase composition and its changes during annealing of plasma-sprayed YSZ, Surface and Coatings Technology 127, pp. 120 - 129, 2000. [ Links ]
[35] Witz, G., Shklover, V. and Steurer, W., Phase Evolution in Yttria-Stabilized Zirconia Thermal Barrier Coatings Studied by Rietveld Refinement of X-Ray Powder Diffraction Patterns, Journal of the American Ceramic Society 90 [9], pp. 2935–2940, 2007. [ Links ]
[36] Di-Girolamo, G., Blasi, C., Pagnotta, L. and Schioppa, M., Phase evolution and thermophysical properties of plasma sprayed thick zirconia coatings after annealing, Ceramics International 36, pp. 2273–2280, 2010. [ Links ]
[37] Lughi, V. and Clarke, D. R., High temperature aging of YSZ coatings and subsequent transformation at low temperatura, Surface and Coatings Technology 200, pp. 1287 – 1291, 2005. [ Links ]
[38] Sheu, T. S., Tien, T. Y. and Chen, I. W., Cubic-to-tetragonal (T) transformation in zirconia-containing systems, Journal of the American Ceramic Society 75, pp. 1108–1116, 1992. [ Links ]
[39] Suresh, A., Mayo, M. J., Porter, W. D. and Rawn, C. J., Crystallite and Grain-Size-Dependent Phase Transformations in Yttria-Doped Zirconia, Journal of the American Ceramic Society 86 [2], pp. 360–62, 2003. [ Links ]
[40] Huang, X., Zakurdaev, A. and Wang, D., Microstructure and phase transformation of zirconia-based ternary oxides for thermal barrier coating applications, Journal of Material Science 43, pp. 2631–2641. 2008. [ Links ]
J.D. Osorio, received a Mechanical Engineering degree in 2008 and a Master's Degree in Engineering in Materials and Processes in 2012, all of them from the Universidad Nacional de Colombia in Medellín, where he worked, from 2007 to 2010, as a research assistant in subjects related with materials characterization, post weld heat treatment in stainless steels, numerical simulation and transport phenomena in thermal barrier coatings for gas turbines applications. He has been awarded with an honorary Mechanical Engineering degree in 2008 and an honors master's thesis in 2010 by the Universidad Nacional de Colombia. From 2011 to 2012 he worked as professor at the Universidad Nacional de Colombia in Medellín, teaching the courses of Dynamics and Engineering Design. He is currently a doctoral candidate in Mechanical Engineering at Florida State University, USA. His research interests are based on thermal barrier coatings, sustainable energy, thermal energy storage and heat transfer optimization of thermal systems.
A. Lopera-Valle, obtained the Mechanical Engineering degree in 2012 from the Universidad Nacional de Colombia in Medellín. In 2010, he joined the Multi-Scale Modeling of Complex Systems: Biophysics and Structured Materials and Tribology and Surfaces research groups. Currently, he is pursuing a MSc in Mechanical Engineering at the University of Alberta, Canada, where he is part of the Advanced Heat Transfer and Surface Technologies research Group. His research interests include: mechanical and thermal modeling of coating systems, biomedical application of polymer, ceramic and composites materials, characterization and application of multilayer coating systems, application of polymers in energy and dissipation processes.
A. Toro obtained his B.S. in Mechanical Engineering from the National University of Colombia in 1997, and a PhD degree from the University of São Paulo in 2001. From 2001 to 2002 he did a postdoc at the Institute for Metal Forming at Lehigh University, USA. He is currently an associate professor of the Department of Materials and Minerals at the Universidad Nacional de Colombia in Medellín and his main areas of interest are industrial tribology, surface analysis and wear-resistant materials.
J.P. Hernandez-Ortiz, received a Mechanical Engineering degree in 1998 from the Universidad Pontificia Bolivariana, where he worked as a research assistant at the Energy and Thermodynamic Institute until 2000. He obtained his PhD degree from the University of Wisconsin-Madison in 2004 in the Department of Mechanical Engineering with a minor in Chemical Engineering. From 2004 to 2007 he did a postdoc in the Department of Chemical and Biological Engineering at the University of Wisconsin-Madison. Currently, he is a Full Profesor in the Department of Materials, Facultad de Minas, at the Universidad Nacional de Colombia in Medellín. He has published more than 50 papers and holds honorary positions at the University of Wisconsin-Madison and the Institute for Molecular Engineering at the University of Chicago. His research interests are based on multi-scale modeling of complex systems for biological and structured materials applications.