Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.186 Medellín jul./ago. 2014
https://doi.org/10.15446/dyna.v81n186.32852
http://dx.doi.org/10.15446/dyna.v81n186.32852
Chemical-mineralogical characterization of copper smelting flue dust
Caracterización químico-mineralógica de polvos de fundición de cobre
Eduardo Balladares a, Ursula Kelm b, Sonia Helle c, Roberto Parra d & Eugenia Araneda e
a Departamento de Ingeniería Metalúrgica, Universidad de Concepción, Chile. eballada@udec.cl
b Unidad de Geología Económica Aplicada (GEA), Universidad de Concepción, Chile. ukelm@udec.cl
c Unidad de Geología Económica Aplicada (GEA), Universidad de Concepción, Chile. shelle@udec.cl
d Departamento de Ingeniería Metalúrgica, Universidad de Concepción, Chile.
e Departamento de Ingeniería Metalúrgica, Universidad de Concepción, Chile. euaraned@udec.cl
Received: September 19th, de 2012. Received in revised form: June 14th, 2013. Accepted: March 5th, 2014
Abstract
In pyrometallurgical processing of copper, 5-10 wt-% of concentrates fed to smelting furnaces are released in the form of flue dust, which contains most of the impurities that pollute products and effluents as well as very significant amounts of copper. Consequently, these dusts cannot be disposed of as an industrial waste and must be treated to recover the copper in order to assure efficient processing. Better process designs needs precise dust characterization. The present study is aimed at generating useful physical, chemical and morphological information of copper smelting flue dust for samples from the gas handling system of a flash smelting furnace. The analyses showed that copper and iron are mainly found in water-soluble phases like chalcantite and chalcocyanite; the water-insoluble fraction contains largely hematite and magnetite; the presence of delafossite is likely. Part of the copper detected in the insoluble fraction is also associated to iron in form of spinel.
Keywords: flue dust, characterization, copper smelting dust.
Resumen
En el procesamiento pirometalúrgico del cobre, hasta 10% de la carga alimentada a los hornos sale de estos en forma de polvo arrastrado por los gases conteniendo la mayor parte de las impurezas presentes en el mineral, así como cantidades significativas de cobre por lo que no pueden ser descartados como residuos industriales y debe tratarse para recuperar el cobre. La conceptualización de nuevos y mejores procesos requiere caracterizaciones de estos materiales más precisas. Se analizaron polvos provenientes de una caldera recuperadora de calor y de un precipitador electrostático, ambos de un horno de fusión instantánea. Las diferentes herramientas analíticas empleadas muestran que el cobre y el hierro se encuentran principalmente en fases solubles en agua tales como chalcantita. La fracción insoluble está formada mayoritariamente por hematita y magnetita, con probable presencia de delafosita. Parte del cobre detectada en la fracción insoluble se asocia al hierro en forma de espinela.
Palabras clave: polvos de fundición, caracterización, fundición de cobre.
1. Introduction
In the pyrometallurgical processing of copper concentrates, the most commonly employed technology is the flash smelting furnace. This type of reactor produces a high strength SO2-bearing gaseous stream that carries an important amount of solid particles outside the furnace. This solid-gas suspension leaves the furnace at high temperature (about 1400 °C), and is treated in a gas-handling system that uses waste boiler heat to separate coarse solids from gas as well as heat recovery to be used in concentrate drying equipment. The gas leaving the waste-heat boiler (WHB) at 350 ºC enters the electrostatic precipitator (ESP) for removal of fine particles leaving a solid-free gas stream suitable for sulfuric acid production in an acid plant.
This solid separated from the gaseous stream constitutes the so-called copper smelting flue dust and comprises a coarse fraction named WHBD (waste heat boiler dust, Dust I) and a fine fraction named ESPD (electrostatic precipitator dust, Dust II).
The composition of these dusts is variable for different operations. The composition depends on the mineralogy of the concentrates, fluxes and circulating material (slag, dust, etc.), and their respective proportions. Thus flue dust is composed of fine particles, fragmented particles and condensed compounds that have been carried out by the gaseous stream.
Recent worldwide primary copper production by pyrometallurgical processes was around 12 million tons a year [1]. The volume of fine solids in the typical smelting-converting operations (only taking into consideration conventional technologies like Outokumpu flash smelting and Peirce-Smith converting) amounts to about 350,000 tons of flue dust each year [2]. These materials are important sources of copper; however, the significant amount of minor elements prevents easy and complete reprocessing in smelting or converting furnaces. Instead, different processes have been developed in each smelter. All these processes take place in aqueous media by either dust leaching or copper and arsenic precipitation followed by a final effluent neutralization step and other unit operations.
Since the mineralogy varies from one concentrate to the next, customized processes should be developed, even when this leads to a complex, high cost, and hard-to-control dust treatment plant that operates as an auxiliary unit to the smelter. Increasingly restrictive environmental regulations complicate design, economics and operation of these treatment units.
Copper smelting flue dusts are defined as "hazardous materials" according to current Chilean environmental regulations, such as DS 148/2003 [3] and DS 185/1991 [4]. This classification of the dusts is mainly due to the high solubility of arsenic compounds in the TCLP (Toxicity Characteristic Leaching Procedure) test and the presence of bismuth and lead. Due to the progressive increase in arsenic content in concentrates and a simultaneous decrease of their copper content, this issue results in a very complex scenario for future smelting operations with respect to environmental issues as well as product quality and overall process cost [5,6].
In order to improve the process, a comprehensive physical-chemical-mineralogical characterization of the flue dusts is needed. Since most characterization requirements are mainly based on current operating practices, the present study uses different analytical techniques to characterize properly copper smelting flue dust in order to have more robust information for decision makers.
2. Background
Flue dust characterization is not a widely studied subject in the literature. The few studies published at present mainly focus on formation of accretions in heat recovery and gas cleaning systems at smelters. Kurosawa et al. [7] reported presence of Fe3O4, PbSO4, Cu2S, Cu2O, As2O5 and PbS in dust collected from the WHB of the Ashio flash smelter as well as vaporization and further condensation of volatile elements downstream. The presence of As(V) is probably due to highly oxidative conditions inside the smelting furnace and ducts for off-gases (between up-take and WHB). Evans et al. [8] investigated the mineralogical composition of the WHB dust and gas stream in the Kidd Creek Mitsubishi smelting furnace, where most of the copper deposits on WHB walls. These authors show the transformation of the dust from oxides to sulfates when passed through the WHB, while other elements, such as zinc and lead, are carried down to the ESP. Kim et al. [9] detected the presence of a wide variety of compounds in the Noranda reactor's ESP dust, which contains mainly PbSO4, other metal sulfates, oxides and zinc ferrites, sulfides and basic copper sulfate (oxysulfates), iron oxides and silicates. Copper bearing phases were associated with iron oxides covered by a lead sulfate layer on the surface.
In the case of a smelting electric furnace, Samuelsson [10] determined that the majority of species correspond to copper, lead and zinc sulfates, cuprous oxide, magnetite and copper-zinc ferrites. In the settling furnace, compounds identified by the same author included iron and zinc oxides, iron and copper sulfides and lead sulfate.
It is important to emphasize that the qualitative and quantitative phase determination of dust depends not only on the compositional characteristics of the concentrate fed into smelting furnaces but also on temperature and oxidative conditions inside the furnace and equipment, which are greatly determined by the reactor type employed.
3. Experimental
The samples (Dust I, Dust II) were obtained from a smelter that operates with a flash smelting furnace followed by Peirce-Smith converters. Two different types of samples were analyzed: the first type comes from the waste heat boiler (WHB) and the second comes from the electrostatic precipitator (ESP). The samples were collected from two parts of the smelter: the WHB and the electrostatic precipitator. In both cases, samples of dust were taken from the discharge screw and from the storage area, using a spatula to collect 1 kg in each case. The spatulas were cleaned using alcohol wipes after each sampling to avoid contamination. The collected material was placed in petri dishes, properly labeled, sealed, and taken to the laboratory. The samples were reduced in the laboratory to 10 g. Since both units (WHB and ESP) operate under different conditions of temperature and oxygen potential, synthetic compounds are not necessarily the same and are present in different proportions.
3.1. Granulometric analysis
Granulometric analyses were performed by laser ray diffraction in a Helos-Succel® equipment that operates with the following settings: Sheat diameter: 20.2 mm; wave length: 0.6328 mm; power: 5mW; deflection: 180º; measuring time: 10 s.
The samples were prepared in a solid-water suspension to avoid particle agglomeration.
Scanning electron microscope (SEM) imaging was carried out on a JEOL JSM 6380 LV.
3.2. Chemical analysis
The chemical analysis was performed by atomic absorption spectrometry in a Hitachi® Z-8100 equipment. Depending on the particular element to be analyzed, a different kind of chemical dissolution technique was used. For Si, Al, Fe, Ca, Pb, Zn, Ni and Co determination the samples were dissolved with an HCl attack, followed by fusion of the insoluble residue with lithium tetraborate. In the case of arsenic and bismuth, these were extracted by using HNO3 at a controlled temperature; for Mo, an oxidative mixture HClO4-H2SO4 was used. Prior to sample dissolution, the presence of minor and mg/kg level trace elements was verified by an X-ray fluorescence spectroscopy scan (Rigaku 3070E WDS, Rh radiation).
3.3 X-ray diffraction analysis
Qualitative XRD was carried out on a Rigaku Dmax C Diffractometer equipped with a horizontal goniometer and operated with Mn-Kb filtered Fe radiation. Samples were scanned (continuous scan) as received and then thoroughly rinsed with distilled water to remove the interference of chalcanthite peaks. Exploratory quantitative XRD analyses were carried out using a URD-6 diffractometer (Seifert-FPM) operated with Co radiation, and a secondary graphite monochromator at 40KV, 30 mA. For Rietveld modeling, step scans were recorded between 5 and 80º 2q, at 0.05º steps and 5 s counting time per step. Rietveld spectral modeling was carried out using BGMN Autoquan software [11].
3.4. Qemscan analysis
Semi-automatic quantitative energy-dispersive scanning electron microscopy (SEM-EDS) analysis were carried out on a QEMSCAN system equipped with four XFlash 275 SDD Bruker detectors at 25 kV and 5 nA. Particle Mineral Analysis mode (PMA) was used at 2.5 mm point spacing resolution to obtain mineral maps and to characterize the As- and Cu-bearing phases using the 4.2 iDiscover-M series software. Due to the material's complexity, a population of 30,000 particles was obtained using Particle Mineralogical Analysis at a resolution of 2.5 pixels. QEMSCAN is capable of categorizing the measured particles based on different mineralogical and metallurgical parameters, including density, locking characteristics, grain and particle sizes.
4. Results and discussion
4.1. Grulometric analysis (grain size and SEM features)
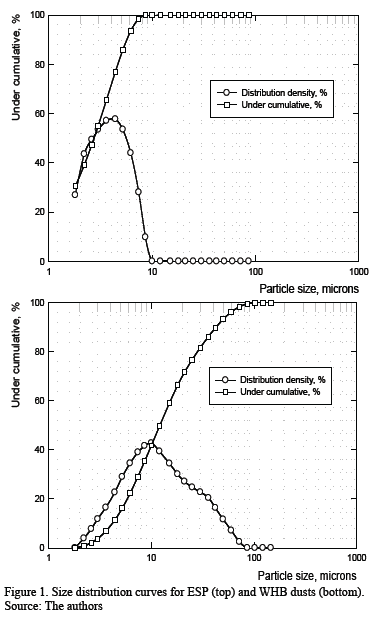
Granulometric analysis was performed on both WHB and ESP dust. The results are shown in Fig. 1 and reveal several interesting features. First, for ESPD, the particle size range is narrow and 100 % of the particles are smaller than 9.3 mm. Second, for WHBD, the particle size range is wider, and the maximum size was 79 mm. These results agree with the operational practice because when the gases (carrying dusts) leave the furnace they first enter the WHB where the gas-solid stream cools down from 1300-1400 ºC to 850 ºC due to air infiltration (at atmospheric temperature). Also, the stream velocity decreases because of the flow’s cross section increases within the WHB, resulting in the collection of coarser particles in the equipment’s lower gas-solid stream cools down to 300 ºC and the finest particles are separated from gaseous stream and retained due to electrostatic and gravitational effects [12].
SEM images allow visual verification of the differences in particles size mentioned earlier. Fig. 1 is clearly related to the measured grain size distribution for WHBD and ESPD, respectively. In particular, ESPD is finer than WHBD due to the loss of coarser dust in the WHB (comparison of Fig. 2 (a) and (c) with (b) and (d)). Another relevant aspect is that the particles from both WHBD and ESPD dust show a spherical or rounded shape rather than edgy or shard-like shapes. This appearance is typical of semi-molten or molten material solidified by cooling in the gas transport system, rather than particulate material reacted in solid state, which has a more angular appearance.
4.2. Chemical analysis
Chemical analyses were performed considering the most important elements present in this kind of materials, namely metallic cations (base metals), sulfur, volatile elements and slagging agents (fluxes). The importance of the first is related to their potential recovery, in particular Cu. On the other hand As, Sb, Bi analyses are needed since the current metal recovery processes involve a great amount of liquid effluents that contain some of these toxic elements and/or produce a solid waste with high mg/kg or % level content of these elements. Furthermore, the content of these elements in the final effluent depends on the dust's initial composition and their form in the feed.
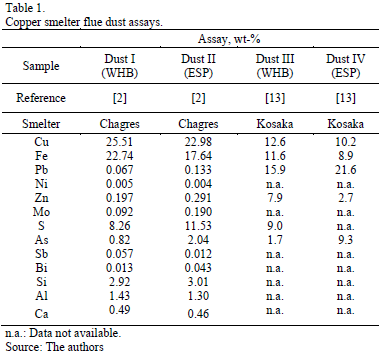
The results of chemical analysis (Table 1, Dusts I and II) account for the most important elements for the smelters in the form of metal for potential recovery of environmentally hazardous elements in effluent treatment and disposal.
Since almost all of the arsenic is present as volatile compounds that condensate at lower temperatures, most of the arsenic appear in the system's cooler (ESP) rather than hotter section. The same situation applies for Dusts III and IV, where the solids' coarse fraction is recovered in the evaporative chamber (1350-850 ºC) and the fine fraction is collected in ESP (near 300 ºC). Since the gases pass through the ESP, vapors containing volatile species begin to condense around solid particles and solidified matte drops, and thus the fine material presents higher content in arsenic (bismuth, antimony, lead and others). Moreover, data in Table 1 illustrate that the chemical compositions of the dust depends on many variables, including the kind of reactor where the smelting and converting processes take place, the specific place of the gas handling system from where dusts are collected, and the chemical-mineralogical characteristics of the concentrate feed. The effect of these variables on the amount and mineralogical features of the dust generated cannot be quantitatively determined, although a qualitative discussion is possible. On the other hand, the results confirm that the dusts contain a significant amount of copper molten and important levels of volatile elements such as As. This is important because the most commonly used hydrometallurgical treatment of the dusts is very sensitive to changes in minor and trace element levels.
Finally, Table 1 shows that both dusts (WHBD and ESPD) contain mainly copper, iron and sulfur. Due to air infiltration in the gas handling system, almost all of the sulfur is associated to copper and iron as sulfates (see XRD results below), resulting from the oxygen and sulfur's partial pressure.
4.3. XRD analysis
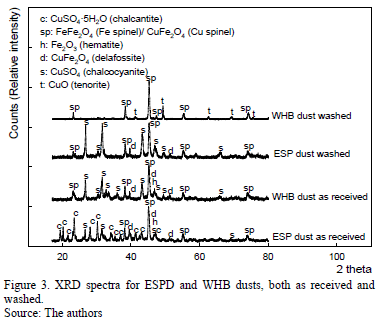
Qualitative X-ray diffraction was performed on samples as received, water rinsed, heated to 150 ºC, 350 ºC and 800 ºC respectively. XRD charts "as received" of Dust I and II are dominated by CuSO4·5H2O (chalcanthite), a spinel phase, e.g., FeFe2O4 (magnetite), minor Fe2O3 and possibly delafossite CuFeO2. Due to the masking effect of chalcanthite on the XRD peaks, we worked with water-rinsed samples as a base for quantitative XRD since the formation of chalcanthite is due to dust storage in environmental humidity and not due to conditions in the WHB and ESP. Heating samples to 150 ºC
helps clear the charts, leaving CuSO4, the spinel phase, Fe2O3 and possibly delafossite. At 350 ºC, the spinel phase dominates, probably magnetite. Due to delafossite, a peak at 28.6 nm is missing, thus not permitting the definite identification of this copper phase. An alternative copper phase would be copper spinel CuFe2O4, although with strong superposition with magnetite. Samples heated to 800 ºC show only the presence of a spinel phase and CuO, where the latter is a product of calcinations in an oxidizing atmosphere.
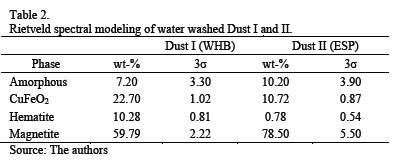
Rietveld spectral modeling (with ZnO as internal standard) was only conducted on water washed samples. Results (see Table 2) corroborate the qualitative observations; however, it is important to note that 7-10 % of the amorphous material probably contain Si and Al as oxides as well as minor elements. The amorphous phase will require further scrutiny in the future.
When comparing the elemental chemical composition of both dusts (WHB and ESP) using AAS (Table 1) with the calculation derived from XRD (Table 2), some discrepancies can be observed regarding major species (Cu, Fe and S). However, these are due to the fact that the elemental analyses shown in Table 1 refer to non-washed dust, whereas for XRD spectral modeling dust has been washed to eliminate chalcanthite spectral overlap (Table 2).
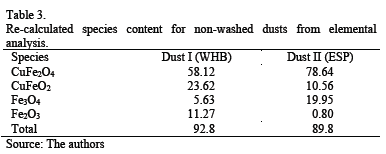
Assuming as a first approximation that during washing only copper sulfate is dissolved (a valid assumption because other soluble species are present in minor proportion), the content of delafossite, hematite and magnetite can be re-calculated using elemental analysis and bringing the species identified by XRD (i.e., 92.8 % for Dust I and 89.8 % for Dust II) to a 100 % basis. The re-calculated content of the mentioned species are shown in Table 3.
These values are obtained by adjusting the CuFeO2/CuFe2O4 and Fe3O4/Fe2O3 ratios (used as parameters), resulting in 0.45 and 0.5 for WHBD and 0.18 and 25 for ESPD, respectively. The comparison of (CuFe2O4+Fe3O4) content (from Table 3) is close to the Fe3O4 content (from Table 2), thus demonstrating the compatible results of chemical and XRD analysis.
4.4. QEMSCAN analysis
Due to the presence of amorphous phases and the need to "clear" the XRD spectrum by water rinsing, QEMSCAN was tested on the coarser dust (Dust I) as an alternative for the quantitative phase characterization of the dusts since this technology of spatial resolved mineralogical semi-automated scanning electron microscopy is now more widely available.
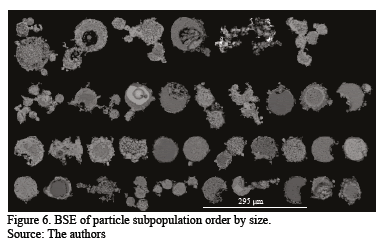
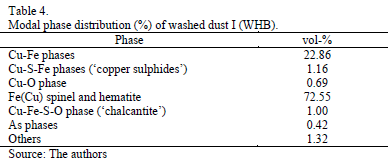
Particles with irregular and mainly rounded shapes were observed. Coarse round particles are surrounded by fine material forming aggregates bonded by a phase likely to be hydrated copper sulfate and other mixed phases. Since the presence of sulfate hydrates implied difficult sample preparation and carbon coating instability, washed samples are preferred for good quality imaging and microchemical data collection. The backscatted electron image (BSE) in Fig. 4 is a size-ranked subpopulation showing the described particle characteristics. Modal phase distribution (Table 3) shows that the main species are copper- and iron-bearing phases making up over 95% of the total population. Only 0.42 % of As phases were identified. Two main groups can be differentiated (Table 3) depending on the Cu and Fe content.
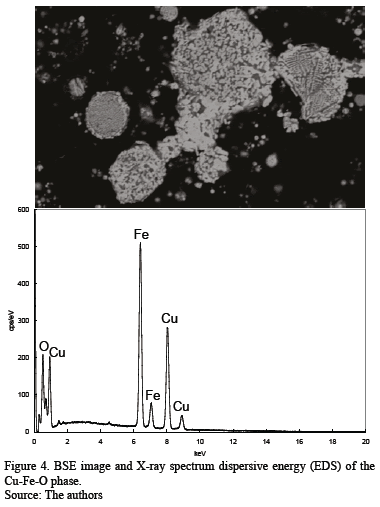
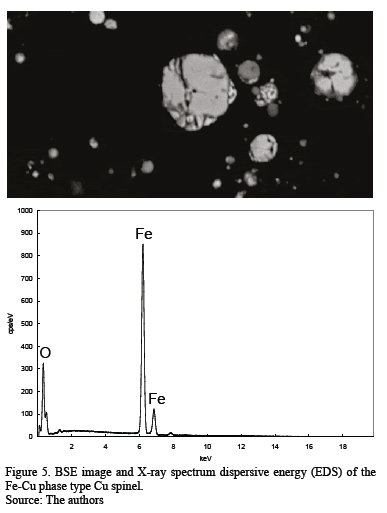
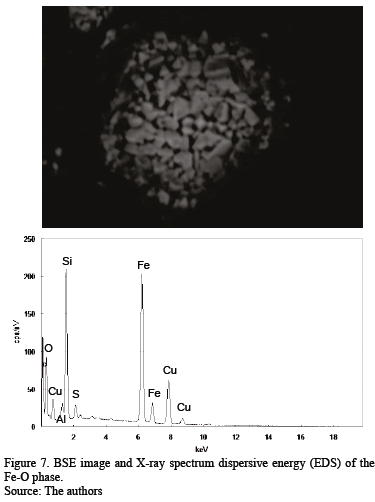
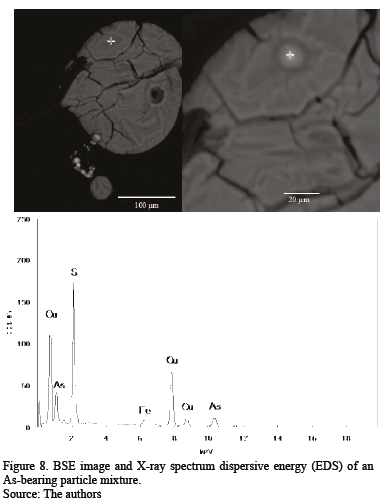
The first Cu-rich group consists of different Cu-bearing phases in even proportions: a Cu-Fe-bearing phase and a Cu-Fe-O phase suggesting a delafossite type, a copper sulfide with low Fe content, and a Cu-O compound, possibly cuprite. The Fe-rich group includes mainly Fe-Cu phases resembling iron-copper spinel and Fe-O phases similar to a hematite/magnetite composition. Some chalcanthite remains from the washing of flue dust or exposure of the sample to ambient humidity; it is associated with the Cu-Fe-S-O phases. Energy dispersive spectra were acquired for some phases in order to corroborate the modal analysis and identification (Fig. 4 - 7). The As-bearing phase (Fig. 8) is unstable under the vacuum conditions used and the incidence of the electron beam causes partial sample volatilization evidenced by clear sample damage.
The results obtained by QEMSCAN performed on a rigorously water washed sample to eliminate most of the chalcanthite, down to 1.00 % (Table 3), are comparable to the calculations of the XRD spectral modeling for Dust I (Table 2) and its recalculated chemical analysis (Table 4).
Since both XRD and QEMSCAN allow semiquantitative-quantitative phase analysis, their application to industrial dusts, characterized by small grain size and even smaller phase size as well as short cooling times (crystallization times of the samples under industrial conditions), will be briefly discussed based on the material analyzed. Desirable aspects for successful Rietveld modeling of XRD spectra are: good crystalline phases with available crystallographic models and an XRD spectrum without the abundant overlap caused by the presence of chalcanthite in this study. Samples need to be analyzed "as received" (bulk), requiring only milling prior to analysis. QEMSCAN record resolution depends on the pixel spacing on the microscope's electron beam source, resolution may be limited to 2 mm. In the case of flue dusts, phases are small (<5 mm), thus limiting the possibility to register single phases without spectral interference from adjacent phases. Reference spectra by quantitative electron microprobe analysis are required for optimum calibration of the phase database. These can at best be obtained from other (coarser) synthetic phases of the same process. However, from the operating conditions, it can only be inferred that similar phases should form in the corresponding dusts. It should also be pointed out that different iron oxides cannot be distinguished. For the material analyzed, a copper sulfate hydrate (chalcanthite) acted as a "glue" between fine particles, complicating phase identification and possibly underestimating the presence of this mineral.
5. Conclusions
Granulometric characterization was successfullly determined for both, WHB and ESP dust samples. The coarser granulometric distribution for the WHB dust respect to the ESP one agrees with that expected from current practice.
The WHB and ESP dusts have similar copper content, specifically 22.98 and 25.51 wt-%, respectively. However, WHB shows higher content of arsenic than ESP, 2.04 compared with 0.82%.
Copper and iron are present mainly in the form of sulfates (water soluble species), and hematite and magnetite (insoluble species). Quantitative XRD for insoluble fraction detected 10.28 and 59.79 wt-% of hematite and magnetite, respectively, in the WHB dust. In the ESP dust, the results are 0.78 and 78.50 wt-%, for the same compounds.
In the case of insoluble copper compounds, results show that delafossite was only tentatively identified due to the absence of one peak. This compound was preliminarily quantified as 22.7 and 10.72 wt-%, for WHB and ESP dust, respectively.
Copper smelting flue dust characterization is a very complex task, requiring systematic efforts combining different techniques to obtain more comprehensive information that can be used to analyze and design dust treatment processes to recover valuable metals and remove toxic elements.
Acknowledgments
The authors thank financial support from DAAD-Conicyt Grant No. 218-13-2007, PBC project PSD-25 and INNOVA Chile project 07CN13PMT-206.
The authors would also like to thank Chagres Smelter and Caletones (El Teniente) Smelter for providing samples and data for the study.
Bibliography
[1] Copper Bulletin and Statistical Yearbook, ICSG, 2009. [ Links ]
[2] Parada, R., Technical manager of chagres smelter, Anglo American Chile. Personal communication. [ Links ]
[3] Biblioteca del Congreso Nacional de Chile, Decreto Supremo DS Nº 148/2003, January 24th, 2004. [ Links ]
[4] Biblioteca del Congreso Nacional de Chile, Decreto Supremo DS Nº 185/1991, April 26th, 1993. [ Links ]
[5] Valenzuela, A., Arsenic management in the metallurgical industry. MSc Thesis, Mines and Metallurgy Department, Laval University, Quebec, Canada, 2000. [ Links ]
[6] Informe País. Estado del Medio Ambiente en Chile 2008, Universidad de Chile, Instituto de Asuntos Públicos, Ed. Centro de Análisis de PolíticasPúblicas, 2010. [ Links ]
[7] Kurosawa, T., Yagishi, T., Togo, K. and Kato ,T., On the several problems of dust in the copper smelting; Transactions of National Research Institute for Metals, 15 (3), 1973. [ Links ]
[8] Evans, J.P., Mackey, P.J. and Scott, J.D., Impact of cooling techniques on smelter dust segregation. In: Smith T.J.A., Newman C.J. ed. Smelter process gas handling and treatment. Warrendale, PA. TMS, 1991, pp. 189-214. [ Links ]
[9] Kim, J.Y., Lajoie, S. and Godbehere, P., Characterization of copper smelter dusts and its effect on metal recovery. In Rao et al. eds. Waste processing and recycling in mineral and metallurgical industries, British Columbia, Canada: CIM, 1995, pp. 221-234. [ Links ]
[10] Samuelsson, C., Controlled dust separation, theoretical and experimental study of the possibilities of controlled dust separation in copper producing processes. Doctoral Thesis Lulea University of Technology, Lulea, 1999. ISSN: 1402-1544. [ Links ]
[11] Bergmann, J., Friedel, P. and Kleeberg, R., BGMN - a new fundamental parameters based Rietveld program for laboratory X-ray sources, it's use in quantitative analysis and structure investigations. Commission of Powder Diffraction, International Union of Crystallography CPD Newslett. 20, pp.5-8, 1998. [ Links ]
[12] Vazán, V., Sarquis, P. y Brandaleze, E., Caracterización de un mineral de cobre en Argentina para la producción de mate, Revista Dyna, 78 (167), pp. 220-228, 2011. [ Links ]
[13] Swinbourne, D.R., Simak, E. and Yazawa, A., Accretion and dust formation in copper smelting-thermodynamic considerations. In: Sulfide Smelting 2002. TMS (The Minerals, Metals and Materials Society), Seattle, Washington, USA, 2002, pp. 247-259. ISBN: 0-87339-525-5. [ Links ]
Eduardo Balladares, received the Bs. Eng in Metallurgical Engineering in 1994, the MSc degree in Metallurgical Engineering in 2004, and the PhD degree in Metallurgical Engineering in 2008, all of them from the Universidad de Concepción, Chile. From 1995 to 1996, he worked as project engineer and from 1997 to 2001, he work at SQM Salar as Plant Chief. From 2002 to 2008, he worked as project engineer. He is currently Assistant professor in the Universidad de Concepción.
Ursula Kelm received her undergraduate degree (Diplom) in geology from Tübingen University, Germany in 1984, followed by a PhD from Bristol University, Great Britain in 1988. Since 1990, she is geologist at the Institute of Applied Economic Geology (GEA), University of Concepción, Chile. Her main areas of interest are gangue mineralogy, in particular clays, as well as the characterization of ultrafine mineral processing materials.
Sonia Helle, received the degree of Bach. in Chemistry in 1975, from the University of Concepción, Chile. From 1973 to now, she works at the University of Concepción. She is full Associate Professor and Director of the Instituto de GeologíaEconómicaAplicada. The fiel of specialization include Geometallurgy, Geochemistry and Atomic Spectrometry Analysis.
Roberto Parra, Graduated from Universidad de Concepción as Metallurgical Engineering in 1991, he obtained a D.E.A. (1992) and a Ph.D. (1998) in Engineering and Material Science, both from Institut National Polytechnique de Grenoble (France). Full professor at the Metallurgical Department of the University of Concepción. The main interests in R&D are Physical Chemistry of High Temperature Processes with special emphasis in copper pyrometallurgy. He is adjunct professor at the Mining School of Oviedo in Spain developing academic activities in the research group of Steelmaking, Metallurgy and Materials and is associated researcher in the group Sustainability on Metallurgical and Steelmaking Processes at CENIM in Madrid (Spain).
Eugenia Araneda, received his degree in Metallurgical Engineering in 1998 and is candidate for the Ph.D. in Metallurgical Engineering at the University of Concepción, Chile. Since 2001 she works as Senior Researcher in the Department of Metallurgical Engineering, University of Concepción, participating in R+D projects in mining with emphasis in electrochemistry and advanced characterization of materials.