Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.187 Medellín set-/out. 2014
https://doi.org/10.15446/dyna.v81n186.38424
http://dx.doi.org/10.15446/dyna.v81n187.38424
Determination of the zeolite optimal diameter for the settlement of nitrifying bacteria in an aerobic bed fluidized reactor to eliminate ammonia nitrogen
Determinación del diámetro óptimo de zeolita para el asentamiento de bacterias nitrificantes en un reactor de lecho fluidizado para eliminar nitrógeno amoniacal
Santiago Pozo-Antonio a
a Department of Natural Resources and Environmental Engineering, University of Vigo, Spain. ipozo@uvigo.es
Received: June 13th, 2013. Received in revised form: May 12th, 2014. Accepted: June 4th, 2014
Abstract
In this work, the determination of the diameter of zeolite as a support for a microbial aerobic fluidized bed reactor is performed. The design of the reactor is recommended by Navarro and Palladino [1]. For the present study, the zeolite is crushed and classified granulometrically. Subsequently, the diameters of 0.5, 1 and 2 mm are arbitrarily chosen for the study of microbial adhesion. After the study of adherence of nitrifying bacteria, the obtained adhesion values for each diameter are not significantly different from each other. However, 1 mm is chosen to achieve higher adhesion values. Subsequently the aerobic fluidized bed reactor proposed by Navarro and Palladino [1] is built with the 1 mm-diameter zeolite. This presented an inlet flow of 1.35 mL.min-1 and a capacity of 8 L. The optimized quantity of zeolite for proper fluidization is 500 g, which is an 8% of total volume of the column. During operation, a good efficiency of reduction of the organic material is observed (50%).
Keywords: nitrification, Nitrosomonas, Nitrobacter, microbial adhesion, fluidization, water.
Resumen
En este trabajo se realiza la determinación del diámetro de zeolita óptimo que es empleado como soporte microbiano en un reactor aerobio de lecho fluidizado. El diseño de dicho reactor es recomendado por Navarro y Palladino [1]. Para el presente estudio, la zeolita es triturada y clasificada granulométricamente. Posteriormente, son escogidos, arbitrariamente, los diámetros 0.5, 1 y 2 mm para realizar el estudio de adhesión microbiana. Tras el estudio de adherencia de bacterias nitrificantes, a pesar de que los valores obtenidos para cada diámetro no presentan diferencias significativas entre sí, se escoge el agregado de 1 mm por conseguir valores de adherencia mayores.
Una vez escogido el diámetro de zeolita se realiza la construcción del reactor aerobio de lecho fluidizado propuesto por Navarro y Palladino [1], con una alimentación de 1.35 mL.m-1 y una capacidad de 8 L. La cantidad de zeolita optimizada para una correcta fluidización es de 500 g, lo que es un 8% del volumen total de la columna. Se observa una buena eficiencia de reducción de la materia orgánica durante el funcionamiento, siendo esta de 50%.
Palabras clave: nitrificación, Nitrosomonas; Nitrobacter; adhesión microbiana; fluidización; agua.
1. Introduction
Reactive forms of common inorganic nitrogen in aquatic ecosystems are ammonium (NH4+), nitrite (NO2-) and nitrate (NO3-). These ions are naturally present in the aquatic environment as a result of atmospheric deposition, surface runoff and groundwater, dissolution of geological deposits rich in nitrogen, biological decomposition of organic matter and nitrogen fixation by certain organisms [2].
Further, humans severely alter the nitrogen cycle by increasing its availability in many regions of the planet as a result of point and diffuse sources of pollution. Widespread pollution produces problems such as toxic algae blooms that after intake through food or water can lead to various physiological disorders and symptoms of intoxication [3-5], eutrophication [6,7], acidification of rivers and lakes with low or reduced alkalinity [8-12], direct toxicity of nitrogenous compounds in aquatic animals [13] and adverse effects on human health [14-16].
In most countries, there are laws and legal regulations establishing limits for the concentration of ammoniacal nitrogen in the wastewater industry such as the Biological Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) [17].
Removal of nitrogen present in the wastewater resulting from domestic and industrial activities is usually carried out combining biological processes of nitrification and denitrification, since costs are lower compared to the physical and chemical processes. Nitrification consists in the biological oxidation (aerobic process) of ammoniacal nitrogen in a first step to nitrite and its oxidation to nitrate, carried out by autotrophic ammonia-oxidizing bacteria (Nitrosomonas) and nitrite-oxidizing (Nitrobacter), respectively [18].
According to the nitrification reaction:
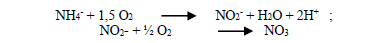
3.43 mg of oxygen (O2) are required to oxidize 1 mg of N-NH3 to nitrite and 1.14 mg of O2 to oxidize 1 mg N-NO2- to N-NO3- [19]. Therefore, concentration of dissolved oxygen in water must be kept around 2 to 3 mg.L-1 for an adequate nitrification [20].
We find various treatment methods that help decrease BOD and COD. Among the most common biological processes, we have fluidized activated sludge, where a granular bed within a column is used. In the column, the lower part has the input of effluent to be treated and recycled effluent and the top part has the treated effluent outlet. In these systems, feed and recycle rates must sustain the fluidization of the bed [1,21] and degradation of organic matter by micro-organisms on the surface of the particles [1, 22-23]. Air injection is essential to get a proper aeration in the system [24]. In these systems, granular activated carbon, sand and clay are used as microbial support [1, 25-26]. The constant levels of biomass must to be maintained. Excess biomass can be removed by friction between particles or transported to a device where the biomass is separated from the particles; these are incorporated back into the bed [27]. Navarro and Palladino proved the effectiveness of an aerobic fluidized bed reactor using as support: granular activated carbon [1]. They achieved an efficiency of biological degradation between 21 and 89 %, using feed rates between 20 and 300 mL.min-1 and recycle flow rates between 1400 and 4800 mL.min-1 and organic loads ranging between 74 and 571 mg.L-1. They determined that higher efficiencies correspond to low flow rates and high organic loads.
In this work, we determine the optimal zeolite size for the correct physiological development of nitrifying bacteria to be used in a subsequent aerobic fluidized bed reactor to remove ammonia nitrogen existing in industrial wastewater.
The building, design and dimensioning of the aerobic fluidized bed reactor have been based on previous works [1, 17]). The optimal size of the zeolite employed as a microbial support was determined by adhesion tests. The whole investigation takes three different stages:
- Crushing and sieving of the zeolite and the choice of the three particle diameters (0.5, 1 and 2 mm).
- -Study of bacterial adherence as a function of the diameter of the particles.
- Manufacturing of the aerobic fluidized bed reactor [1]. The diameter of the zeolite used as microbial support was the one that corresponds to the best adherence results from the previous stage.
To ensure a proper implementation of the fluidized bed aerobic reactor a number of biological, chemical and physical parameters such as temperature, pH, microbial support, volume, feeding rate, retention time, and the reactor dimensions and configuration must be taken into account [28].
There are nitrifying microorganisms showing activity in a temperature range from 5°C to 42°C [29]. Most often, the optimum operating temperature is in the range from 28 to 36°C [29]. Temperature effects on the kinetics constant is very important, thus 1ºC change between 25 and 26°C gives rise to a 9.5% increase of the growth rate of Nitrosomonas and 5.9% of the Nitrobacter [30].
Alkaline media are more favourable for nitrifying bacteria with optimal levels of pH between 7.5 - 8.5 [31]. The pH value affects the environmental conditions for a favourable physiological development of microorganisms, and also has a great influence on the inhibition degree of the nitrifying bacteria. It was found that ammonium produces the inhibition of Nitrobacter [31]. Nitrifying bacteria are sensitive to multiple substances (heavy metals, organic compounds, free ammonia, etc.). These substances can interfere with the cell metabolism, reducing the rate of formation of intermediates compounds [32].
For these bacteria to develop physiologically, a proper support material should be chosen to take into account the biological process itself, the equipment size and experimental conditions that bacteria will find. In this case, the preferred zeolite particle size for the fluidized bed reactor was between 0.1 and 1 mm, as smaller sizes difficult operations with the reactor [33]. Most of the materials, such as sand and clay with irregular shapes and sizes, were chosen to take into account that they were cheap and readily available.
One of the most important variables in reactor design is the density of the aerobic fluidized bed material, as it affects the bed hydrodynamics and has a direct effect on power consumption [34]. Density affects the bed fluidization, so when particle density approaches the corresponding value for the fluidizing liquid, velocities for minimum fluidization conditions and for a 20% expansion, become close. It must be noted that particles should not be fragile as because of their continuous movement they can collide and become fragmented, changing their fluidizing characteristics and thus making even more difficult the control of the bed expansion. In this sense, there are works that use active carbon as the support, with a density lower than that of the natural zeolite [1].
One advantage of fluidized bed reactor is the large surface available for adhesion, where the biofilm can grow. It was determined that optimum pore size is about five times the dimension of the cells, that leave room for two adhered cells and two more cells growing by fusion and some free space between them for transferring substrates [35].
2. Materials and methods
2.1. Crushing and sieve analysis
Upon receipt of the zeolite from the supplier, the grinding was started to obtain the required diameters (0.5, 1, 2 mm) using a crusher (DECO BH41A) and a bar mill (DECO 7997B). Then ASTM Sieves numbers 11, 16, 20, 30 and 35 were used to sieve the zeolite.
2.2. Microbial adhesion
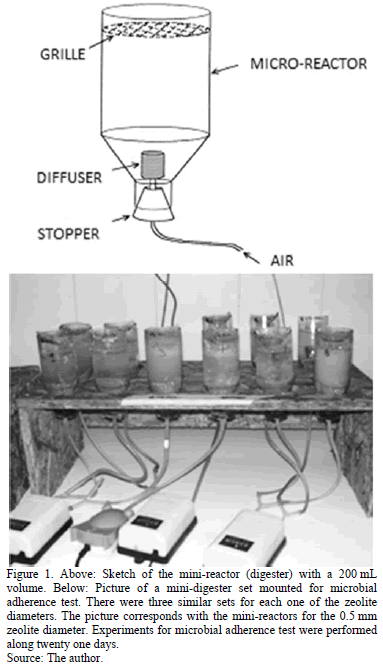
For the microbial adhesion tests, assemble of 16 mini digesters were used with a volume of 200 mL, for each one of the zeolite diameters (Fig. 1).
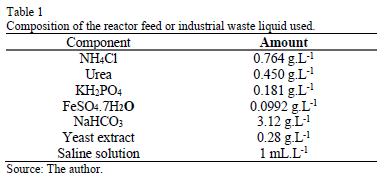
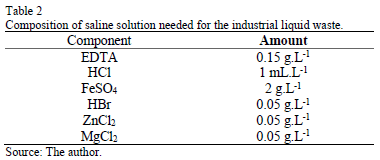
Each mini-digester contained 40 g of zeolite, 100 mL of liquid industrial waste and 72 mL of sludge. Aeration was performed with dual output aerating pumps (RESUN AC 6600, 45L, 220V). The industrial waste feeding liquid to the reactor corresponds to a synthetic solution, whose composition was obtained from previous works [36]. Table 1 details the composition of the feeding liquid and Table 2 shows that of the saline solution used to prepare the synthetic industrial waste liquid.
The mini-reactors were inoculated with microorganisms (nitrifying bacteria) from an activated sludge plant, which was in a process of recovery. Prior to their employment in the mini-reactors, they were subjected to the following characterization (Table 3):
- pH determination using a Crison pH 25 pH-meter.
- Total Suspended Solids (TSS) measurement (Standard Methods 2540 D, drying 103 - 105°C) [37].
- Volatile Suspended Solids (VSS) measurement (Standard Method 2540 E, drying from 103 to 105°C and ignition till 550°C) [37].
- Total Chemical Oxygen Demand (CODT) determination [37].
Once the mini-digesters were filled and the aeration pumps connected, the system was allowed to evolve for five days. After this initial time zeolite and liquid samples were taken every two or three days. Mini-digesters were grouped in pairs, for each zeolite diameter. Each sampling day, the zeolite and liquid of both mini-digesters from one of the pair for the three different zeolite diameters were analyzed. Most assays were done three times in order to have well defined average values. This method allows considering that the mass of the mini-digester was kept constant during the whole sampling time. The whole experiment ran for three weeks.
The liquid part of each sample was characterized by measuring the pH, TSS, VSS and CODT following the above-mentioned standards.
Adhesion tests were performed with the zeolite extracted from each mini-reactor. The extracted zeolite was placed on a filter paper on a capsule and introduced into a hot oven for six hours to ensure complete drying of the support. To determine the mass of the adhered solids the following expression can be applied:
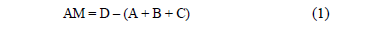
where:
AM: adhered solids mass (g)
A: mass of the dry filter paper, where the zeolite is placed (g)
B: mass of the supporting capsule (g)
C: mass of zeolite added initially to the mini-digesters (g)
D: dry mass of the capsule with the zeolite and filter after six hours into the heating oven (g).
2.3. Implementation of aerobic fluidized bed reactor
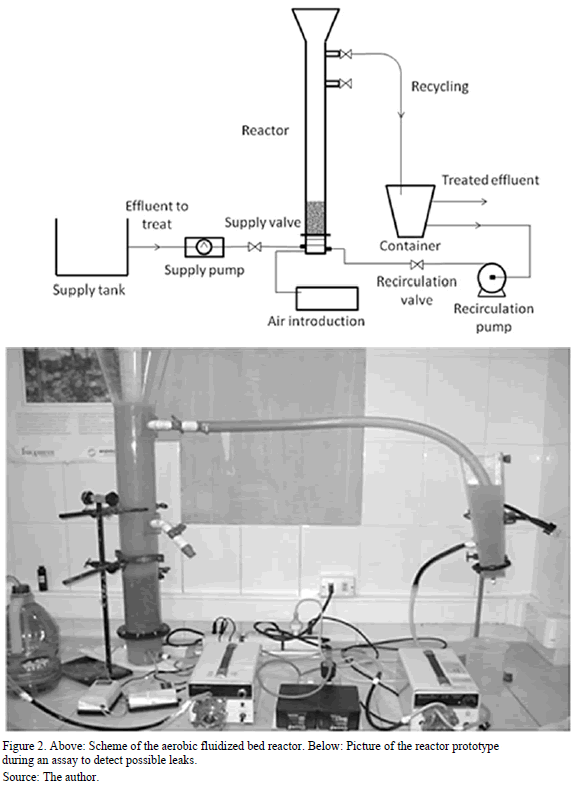
After the adhesion test, the construction of the aerobic fluidized bed reactor based on the construction of Navarro and Palladino was performed using the zeolite of the most adequate diameter [1]. The reactor consisted of an acrylic column 10 cm in diameter and 1.05 m height. The final 20 cm corresponded to an inverted truncated cone with a 20 cm diameter base (Fig. 2). This design was chosen in order to prevent particles escaping from the reactor. The acrylic column had two side outlets located at 25 and 70 cm from the top. Both side outlets with a 2.5 cm diameter possessed outlet valves and hoses with the same diameter. The plate that support the filler was composed of a stainless steel mesh placed 10 cm above the base. Below the plate there were feeding inlets for the effluent to be treated and recycling. Above these two inlets two cross-placed diffusers ensured an even air distribution. The installation also had a 20 cm high pyramidal settler with a weir at the top for discharge of treated effluent and a bottom outlet for recycling. A purge was also available for use if necessary. The supporting material used was zeolite of the chosen diameter. This was loaded onto the column to achieve a fixed bed height of 15 cm.
This height was set so that a significant liquid volume was above the bed of particles, thereby enabling work with different fluidizing speeds and thus different bed expansions. Two peristaltic pumps completed the system, for a constant feed rate and an optimum recirculation.
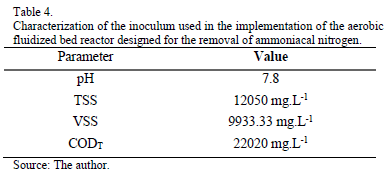
The reactor was filled with 500 g of zeolite, 4783 mL of liquid industrial waste and 2416 mL of sludge. The liquid industrial waste had the same composition as that used in the mini-digesters (Tables 1 and 2). The sludge was from another source, so it was again subjected to the analysis of pH, TSS, VSS and CODT [38]. Results are shown in Table 4.
2.3.1. Tuning of the reactor
A nitrogen load speed (NLS) of 0.11 kgN.m-3.d-1 [39] and a Total Kjeldahl Nitrogen (TKNi) of liquid industrial waste of 452 mg.L-1 were used [36] that resulted in a Hydraulic Residence Time (HRT) of 98 hours.
A first run was performed with water to verify the state of the seals and the general operation of each system component (Fig. 2). Aeration equipment is critical for this type of reactors as to achieve the expected efficiency a proper air distribution is needed.
Once confirmed that no leakage or conduction problems occurred, 8 L of the synthetic industrial waste liquid were introduced within the column. Then the structure was mounted and the system switched on. Firstly, with a total recirculation and later, when the recirculation rate was chosen, the feeding was started. The feeding pump was connected to a timer to achieve the necessary inflow. The NLS and TKNi experimental values corresponded to an inlet flow of 1.35 mL.min-1, so the pump was calibrated to that flow rate.
The reactor was in operation for 21 days (like the mini-digesters). Different control parameters were measured at the beginning and end of the operation. These parameters are: organic load in the effluent input (CODTent) and output (CODTexit), pH of the treated effluent, VSS, TSS and microbial adhesion).
3. Results and discussion
3.1. Crushing and sieve analysis
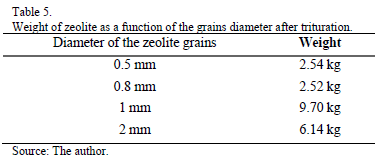
As shown in Table 5, after crushing and sieving enough zeolite for each of the expected sizes (0.5, 1 and 2 mm) was obtained.
The optimum crushing and sieving times for the zeolite was determined to be 10 min and 8 min, respectively.
3.2. Microbial adhesion
Graphs and results for each performed analyses are presented below.
3.2.1. pH behavior
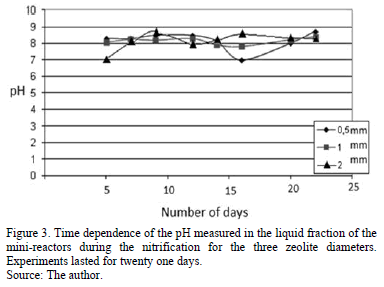
Fig. 3 shows the pH as a function of time (in days). To avoid inhibition the pH value must be kept in the range from 7.5 to 8.5, as alkalinity of the system prevent the pH lowering caused by the nitrification [31].
It is observed that the pH was kept constant along the experiment time for 1 and 2 mm diameter zeolite samples. However for the 0.5 mm diameter case, pH was decreased in the sixteenth day till twentieth. This is a value beyond the optimal range for the survival of aerobic nitrifying bacteria. However, this is not an acidic enough pH to avoid the life of microorganisms, so this diameter zeolite may perform its metabolic functions, but not optimally.
3.2.2. Organic filler
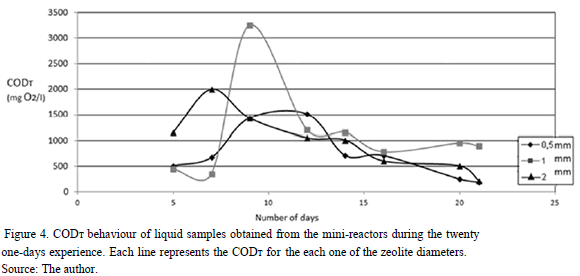
CODT results are shown in Fig. 4. It can be seen that there was an almost complete biodegradation of organic matter in all samples for the different diameters, being 99.3, 95.0 and 98.6 % for the 0.5, 1 and 2 mm diameters, respectively. The best removal was obtained for the 0.5 mm diameter case; however, values are so close that statistical analysis determined that there are no significant differences among them (P=1>0.05).
3.2.3. Suspended Solids
TSS and VSS refer to the microorganisms found in industrial waste liquid, i.e. they are not adhered to the interstitial spaces of the zeolite granules. TSS comprises both microorganisms and every substance that is suspended in the liquid industrial waste; however, VSS refers only to the microorganisms. As a consequence TSS values must always be greater than VSS values and both must show a similar behaviour.
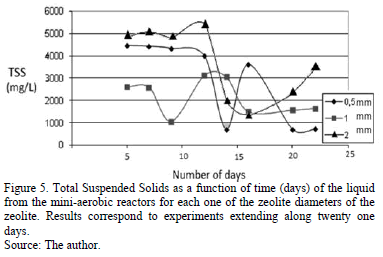
Fig. 5 shows the TSS time dependence for the three zeolite diameters used in the experiments.
As can be seen, for the 0.5 mm zeolite diameter, TSS values remains nearly constant from the fifth to the ninth day; later, on the fourteenth day, an abrupt decrease from 3990 to 700 mg.L-1 was observed. This result is in accordance with the adherence maximum observed for the same day. Then there is a slight increase in the seventeenth day followed by a fall back to values similar to the one of the fourteenth day. A similar behaviour was observed for the 2 mm zeolite diameter case, the TSS was initially roughly constant and then an abrupt decrease from 5403 mg.L-1 (eleventh day) to 1376 mg.L-1 (thirteenth day) was observed. This decrease was followed by a slight increase following days. This increase is associated to the cryptic growth stage of the microorganisms natural cycle (growth curve), which takes place after the death phase. For the 1 mm diameter case, a minimum for the TSS value (1060 mg.L-1) was observed the ninth day. Further, TSS value increased to 3120 mg.L-1 due to the above mentioned cryptic growth stage. At the experiment end TSS values were constant.
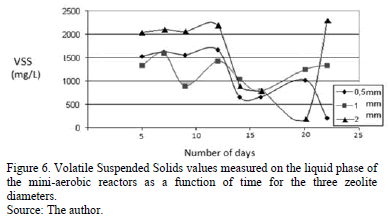
Fig. 6 shows the time dependence of VSS. As indicated, they have a behaviour similar to TSS values, but the VSS values are always smaller than the TSS ones. For the 0.5 mm zeolite diameter the initial TSS values remain nearly constant. On the twelfth day, VSS began to decrease to a minimum of 530 mg.L-1 that was reached on the fourteenth day. A similar evolution was observed in the 2 mm zeolite diameter case. In this case, VSS also decreased after the twelfth sampling day, but the values were different in magnitude and the decreasing behaviour was extended to the twentieth day where a minimum of 200 mg.L-1 was reached. The final value corresponding to a drastic increase till 2300 mg.L-1 is due to errors in the data collection and sampling. Finally, in the case of the zeolite with 1 mm diameter, VSS values showed a continuous fluctuation along the whole experiment time, with a minimum of 790 mg.L-1 on the sixteenth day and a slight increase until the end of the experiment.
3.2.4. Microbial adhesion
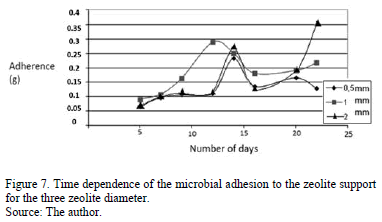
Fig. 7 shows the behaviour of nitrifying bacteria adhesion on the zeolite support. Unlike both Volatile and Total Suspended Solids, there is an initial increasing trend of the adhesion, for the first days of the experiment, that finished on the eleventh day for the 1mm zeolite diameter of 1 mm and the thirteenth for 0.5 and 2 mm zeolite diameter, where maximum values were reached. Later (on the fourteenth day) and for the three cases, a decreasing behaviour was observed until curves reached a nearly constant value at the experiment end.
The initial region of the adhesion increase, shown in Fig. 7, is associated with nitrifying bacteria growth due to the presence of nutrients in the synthetic liquid industrial waste. This growth extends until not enough zeolite interstitial
space was available for the bacteria. This state corresponds with the maximum of the adhesion curves. Then the bacteria far away form interstitial spaces dissociated from the zeolite and, as a consequence, the TSS value increased while adhesion decreased. The adhesion maximum was achieved for the 1 mm zeolite diameter, with a value of 0.2891 g of nitrifying microorganisms adhered. However, similar values were obtained for the 0.5 and 2 mm zeolite diameters, 0.2329 and 0.2729 g, respectively. This similarity in the results is in accordance with the statistical analysis, that yielded P> 0.05, which indicate that the zeolite diameter was not a determining parameter for the microbial adhesion (see explanation in next section).
Thus a 1 mm zeolite diameter was used in the implementation and operation of the fluidized bed aerobic reactor.
The statistical analysis performed, with the Minitab software package, on the amount of mass attached to the zeolite contained into each mini-reactor indicated that there are no significant differences between the three diameter values, as P = 0.325>0.05. Data differences were determined using one-way ANOVA. However, experience showed that notwithstanding similar in a statistical sense, the best adhesion was obtained for 1 mm diameter and therefore this was the chosen size to be employed in the reactor construction.
3.3. Tuning of the aerobic fluidized bed reactor
As a general fact, one can conclude that the zeolite was a good support for microorganism population, in this case of nitrifying bacteria for the removal of nitrogen. That made it useful to be included in fluidized bed reactors.
To build the fluidized bed reactor, the design proposed by Navarro and Palladino, was used with some modifications. First modification related to the employed microbial support [1]. There activated carbon support was used, with a density ranging from 0.3 to 0.7 g.cm-3. In this work, the zeolite used had a density of 2 g.cm-3, leading to a lower amount used in the reactor as compared to activated carbon. Therefore, the power of the diffusion pump was enough to overcome the gravitational forces acting on the particles to achieve the fluidization of the system entirely. As Navarro and Palladino showed [1], the volume of activated carbon is 50%, but in this study the optimal volume of zeolite is 8%.
In the Table 6, the parameters which were measured after 21 days of operation.
If we compare Tables 4 and 6, we can observed that the pH is maintained constant during 21 days. The COD experimented an important decrease from 22020 to 10050 mg.L-1. As Navarro and Palladino [1] obtained, the biological degradation using this kind of aerobic fluidized bed reactor is acceptable; we have obtained a biological degradation of 50%.As they affirmed, with higher CODTent, the degradation efficiency is better.
4. Conclusions
The optimum crushing and sieving times for the zeolite was determined to be 10 min and 8 min, respectively. The sieving process to obtain the 1 mm diameter zeolite was shown to be the more efficient.
There is not significant differences in the measured microbial adhesion for the three diameters (0.5, 1 and 2 mm), thus the zeolite diameter in this range is not a determining parameter to adhesion of nitrifying microorganism colonies.
The treatment system of liquid effluents designed by Navarro and Palladino was tested, changing the activated carbon by zeolite as a microbial support. Results indicated an organic degradation of 50%. Microbial degradation is acceptable.
In response to the experience, a constant control of the reactor is required to achieve a good oxygenation.
The reactor, designed by Navarro and Palladino [1], has proven to be a well suited system for removing of ammonia nitrogen that is present in industrial waste liquids.
In conclusion, operating with a feed rate of 1.35 mL.min-1 and a capacity of 8 L, with an organic load input of 22020 mg.L-1, a reduction efficiency of organic matter of 50% is achieved.
The higher efficiencies correspond to low flow rates and high organic loads.
Acknowledgments
Author wants to give thanks to University of Vigo for the financial support given.
References
[1] Navarro, A.F. and Palladino, L.A., Degradación de efluentes líquidos mediante lechos fluidizados. Información Tecnológica 20 (5), pp. 13-18, 2009. [ Links ]
[2] Abel, P.D., Water Pollution Biology. 2nd edition, Taylor & Francis Publishers, London. 2000. [ Links ]
[3] Hitzfeld, B.C., Höger, S.J. and Dietrich, D.R., Cyanobacterial toxins: Removal during drinking water treatment and human risk assessment. Environ. Health Perspectives 108 (SUPPL.), p. 113-122, 2000. [ Links ]
[4] Van Dolah, R.F., Chestnut, D.E., Jones, J.D., Jutte, P.C., Riekerk, G., Levisen, M. and McDermott, W., Environmental Monitoring and Assessment 81, pp. 85-95, 2003. [ Links ]
[5] Newcombe, G., Chorus, I., Falconer, I. and Lin, T-F., Cyanobacteria: Impacts of climate change on occurrence, toxicity and water quality management. Water Research (Editorial) 46, pp. 1347-1348, 2012. [ Links ]
[6] Iosip, A., Dobon, A., Hortal, M. and Bobu, E., The influence of contaminants in the environmental impact of recovered paper: A life cycle assessment perspective. Int J Life Cycle Assess. 17, pp. 1050-1058, 2012. [ Links ]
[7] Karydis, M. and Kitsou, D., Eutrophication and environmental policy in the Mediterranean Sea: A review. Environ Monit Assess. 184, pp. 4931-4984, 2012. [ Links ]
[8] Caraballo, M.A., Macías, F., Rötting, T.S., Nieto, J.M. and Ayora, C., Long term remediation of highly polluted acid mine drainage: A suitable approach to restore the environmental quality of the Odiel river basin. Environmental Pollution 159, pp. 3613-3619, 2011. [ Links ]
[9] Palmer, S.C.J., van Hinsberg, V.J., McKenzie, J.M. and Yee, S., Characterization of acid river dilution and associated trace element behavior through hydrogeochemical modeling: A case of the Banyu Pahit River in East Java, Indonesia. Applied Geochemistry 26, pp. 1802-1810, 2011. [ Links ]
[10] Dos Santos, C., Bernusso, V.A., Sobreiro, H., Gaeta, E.L. and Fernandes, M.N., Biomarket responses as indication of contaminant effects in Orechromis niloticus. Chemosphere 89, pp. 60-69, 2012. [ Links ]
[11] Nystrand, M.I., Österholm, P., Nyberg, M.E. and Gustafsson, J.P., Metal speciation in rivers affected by enhanced soil erosion and acidity. Applied Geochemistry 27, pp. 906-916, 2012. [ Links ]
[12] Jardine, T.D., Kidd, K.A. and Rasmussen, J.B., Aquatic and terrestrial organic matter in the diet of stream consumers: implications for mercury bioaccumulation. Ecological Applications 22 (3), pp. 843-855, 2012. [ Links ]
[13] Mebane, C.A., Dillon, F.S. and Hennessy, D.P., Acute toxicity of cadmium, lead, zinc and their mixtures to stream-resident fish and invertebrates. Environmental Toxicology and Chemistry 31 (6), pp. 1334-1348, 2012. [ Links ]
[14] Fewtrell, L., Drinking-Water Nitrate, Methemoglobinemia and global burden of disease: A discussion. Enviromental Health Perspectives 112 (14), pp. 1371-1374, 2004. [ Links ]
[15] Kay, D., Bartram, J., Prüss, A., Ashbolt. N., Wyer, M.D., Fleisher, J.M., Fewtrell, L., Rogers, A. and Rees, G., Derivation of numerical values for the World Health Organizations guidelines for recreational waters. Water Research 38, pp. 1296-1304, 2004. [ Links ]
[16] Camargo, J.A., and Alonso, A., Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environmental International 32, pp. 831-849, 2006. [ Links ]
[17] Bertola, N., Palladino, L., Bevilacqua, A. and Zaritzky, N., Optimisation of the design parameters in an activated sludge system for the wastewater treatment of a potato processing plant. Journal of Food Engineering 40, pp. 27-33, 1999. [ Links ]
[18] Daims, H., Maixner, F. and Schmid, M.C., The Nitrifying microbes: Ammonia oxidizers, nitrite oxidizers and anaerobic ammonium oxidizers. In: Nielsen, P., Daims, H. and Lemmer, H. FISH Handbook for biological wastewater treatment: Identification and qualification of microorganism in activated sludge and biofilms by FISH. London, United Kingdom, Ed. IWA, 2009, pp. 9-17. [ Links ]
[19] Fernández-Polanco, F., Villaverde, S. and García, P.A., Temperature effect on nitrifying bacteria activity in biofilters: Activation and free ammonia inhibition. Water Sci Technol., 30 (11), pp. 121-130, 1994. [ Links ]
[20] Hanaki, K., Wantawin, C., Ohgaki, S. Nitrification at low levels of dissolved oxygen with and without organic loading in a suspended-growth reactor. Water Research 24 (3), pp. 297-302, 1990. [ Links ]
[21] Yang, W.C., Handbook of Fluidization and Fluid Particle System, Ed. by. CRC Press. N.Y. 2003. [ Links ]
[22] Shieh, W.K., Sutton P.M. and Kos P., Predicting reactor biomass concentration in a fluidized bed system, Journal WPCF:, 53 (11), pp. 1574-1684, 1981. [ Links ]
[23] Wang, X.C., Yuan, H.L., Liu Y.J. and. Jin, P.K., Fluidized pellet bed birreactor; a promising technology for onsite wastewater traetment and reuse, Water Science and Technology, 55, pp. 59-67, 2007. [ Links ]
[24] Rajasimman, M. and Karthikeyan, C., Aerobic digestion of starch wastewater in a fluidized bed bioreactor with low density biomass support, Journal of Hazardous Materials, 143, pp. 82-86, 2007. [ Links ]
[25] Nicolella, C., van Loosdrecht, M.C.M and Heijnen, J.J., Wastewater treatment whit particulate biofilm reactor, Journal of Biotechnology: 80, pp. 1-33, 2000. [ Links ]
[26] Gómez, C. y Lopera, E., Evaluación experimental de las velocidades mínimas de fluidización para mezclas de carbón ripio y residuos agrícolas Colombianos, DYNA (Colombia), 78 (169), pp. 105-112, 2011. [ Links ]
[27] Perry R.H. and Green, D., Perry's Chemical Engineering Handbook, 7th edición, Sec. 25, McGraw Hill Companies, Inc. United State of America, pp. 75 - 76, 1999. [ Links ]
[28] Palm, J.C., Jenkins, D. and Parker, D.S., Relationship between organic loading, dissolved oxygen concentration and sludge settleability in the completely-mixed activated sluge process, Journal of the Water Pollution Control Federation 52, pp. 2484-2506, 1980. [ Links ]
[29] Gerardi, M., Nitrification and denitrification in the activated sludge process. Wiley- Interscience, New York, 2002. [ Links ]
[30] Sharma, B. and Ahlert, R.C., Nitrification and nitrogen removal. Water Research 11, pp. 897-925, 1977. [ Links ]
[31] Anthonisen, A.C., Loehr, R.C., Prakasam, T.B.S. and Srinath, E.G., Inhibition of nitrification by ammonia and nitrous acid, Journal of the Water Pollution Control Federation 48 (5), pp. 835-852, 1976. [ Links ]
[32] Garrido, J.M., Nitrification and denitrification of wastewater with high concentrations of formaldehyde and urea. PhD dissertation, Santiago de Compostela University, Spain, 1996. [ Links ]
[33] Cunningham, A.B., Bouwer, E.J. and Characklis, W.G., Biofilms in porous media. In: Biofilms. Characklis, W.G. and Marshall, K.C. eds. Wiley and Sons, New York, 1990. [ Links ]
[34] García, C.D. and Buffiere, P., Anaerobic digestion of wine distillery wastewater in Down-Flown fluidized Bed. Elsevier Science 38, pp. 3593-3600, 1998. [ Links ]
[35] Messing, R.A. and Oppermann, R.A., Pore dimensions for accumulating biomass. I Microbes that reproduce by fission or by budding. Biotechnology Bioeng 21, pp. 49-58, 1979. [ Links ]
[36] Dal Pozzo, L.A., Design of a joint-reactor for nitrificación-denitrificaction biological. PhD dissertion, Federico Santa María University, Chile, 1999. [ Links ]
[37] American Public Health Association, Standard Methods for the Examination of Water and Watewater. Water Environment Federation, Washington, DC. 1998. [ Links ]
[38] Arnold, E.G., Lenores, S.C., Andrew, D.E. Standard Methods for the Examination of Water and Watewater. American Public Health Association. Washington, DC. 1992. [ Links ]
[39] Muñoz, M.A., Construction of a joint-reactor of nitrification-denitrification. Degree dissertation, Federico Santa María University, Chile, 2006. [ Links ]
S. Pozo-Antonio, received a Bs. Eng. in Mining in 2009, a MSc. degree in Environmental Technology in 2010, and his PhD. degree in Environmental Technology in 2013, all of them from the University of Vigo, España. From 2010 to 2013, he worked in the Department of Natural Resources Engineering and Environment in University of Vigo in several projects related with mining and Conservation and Restoration of Built Heritage. From 2013-2014 he got an internship in Getty Conservation Institute in Los Angeles (USA). His research interests include: cultural built heritage, mining techniques, landscape management and landscape assessment.