Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.187 Medellín set-/out. 2014
https://doi.org/10.15446/dyna.v81n186.38922
http://dx.doi.org/10.15446/dyna.v81n187.38922
Vinasse treatment by coupling of electro-dissolution, hetero-coagulation and anaerobic digestion
Tratamiento de vinazas acoplando electrodisolución, heterocoagulación y digestión anaerobia
Olga Lucía Paz-Pino a, Luz Edith Barba-Ho b & Nilson Marriaga-Cabrales c
a Departamento de Química, Universidad del Valle, Colombia. olgalu.pp@gmail.com
b Escuela de Ingeniería de los Recursos Naturales y del Ambiente, Universidad del Valle, Colombia. luz.barba@correounivalle.edu.co
c Escuela de Ingeniería Química, Universidad del Valle, Colombia. nilson.marriaga@correounivalle.edu.co
Received: July 22th, de 2013. Received in revised form: May 12th, 2014. Accepted: July 16th, 2014
Abstract
The distillery vinasse is the most important liquid effluent of the ethanol production process and it is characterized by a high chemical oxygen demand (COD) and high biochemical oxygen demand (BOD); besides its acid pH and dark brown color. Phenol content in vinasse causes inhibitory effects on anaerobic digestion processes and has an adverse environmental impact. An alternative for treating vinasse from distilleries by using the electro-dissolution of iron and a heterocoagulation stage with Ca(OH)2 as a pretreatment was evaluated. Removals of 92% in phenols and 52% in chemical oxygen demand (COD) were obtained. Then, the pretreated vinasse was undergone to an anaerobic digestion process. Global removals to around 93% in biochemical oxygen demand (BOD5), 83% in COD, and over 99% in total phenols were obtained. Finally, an eco-toxicological evaluation using Daphnia pulex was carried out, and it was found that the coupling treatment increased the medium lethal concentration (LC50) to around 190%.
Keywords: Vinasse, phenols; electro-dissolution; heterocoagulation; anaerobic process; Daphnia pulex.
Resumen
La vinaza es el principal efluente del proceso de producción de etanol y se caracteriza por su alta demanda química de oxígeno y alta demanda biológica de oxígeno; además, un pH ácido y por su color marrón oscuro. El contenido de fenoles en la vinaza ocasiona efectos inhibitorios en los procesos de digestión anaerobia y presenta un impacto ambiental adverso. Se evaluó un tratamiento alternativo de vinazas de destilería empleando como pretratamiento una etapa de electrodisolución de hierro y heterocoagulación con Ca(OH)2 logrando reducciones del 92% en el contenido de fenoles y 52% en la demanda química de oxígeno (DQO). Posteriormente, la vinaza pre-tratada se sometió a digestión anaerobia alcanzando reducciones globales cercanas al 93% en demanda bioquímica de oxigeno (DBO5), 83% en DQO y mayores al 99% en el contenido de fenoles. Finalmente, se llevó a cabo una evaluación toxicológica empleando Daphnia pulex y se encontró que con el tratamiento acoplado la concentración letal media (LC50) se incrementó cerca de 190%.
Palabras clave: Vinazas; fenoles; electrodisolución; heterocoagulación; biodegradabilidad anaerobia; Daphnia pulex.
1. Introduction
Vinasse is the main liquid effluent in ethanol-producing industry which generates between 9 and 14 liters of this effluent for each liter of ethanol produced. This wastewater is characterized by a high chemical oxygen demand (COD) ca 80,000-100,000 mg L-1 and high biochemical oxygen demand (BOD) ca 40,000-50,000 mg L-1; besides its low pH, strong odor, and dark brown color [1]. This effluent presents a high dissolved organic matter content, as well as a considerable quantity of inorganic salts composed by chlorides, sulfates, phosphates, calcium, magnesium, and potassium [2]. Vinasse is potentially toxic because of its bio-recalcitrant substance contents, such as phenolic compounds, and pigments like melanoidins, which can inhibit the activity of microorganisms present in water sources [3]. Although, liquid vinasse has been used as a fertilizer to improve soils [4]; it also presents adverse environmental impacts, such as salt-enriching of soil, and nutrient runoffs in rainy seasons.
The presence of phenolic compounds deserves special attention because of their high toxicity. They are known as a kind of pollutants to be prioritized by USEPA [5]. On the other hand, phenol-compound toxicity has been studied by using anaerobic and aquatic organisms [6, 7].
Several technological processes for treating vinasse have been researched; physicochemical treatments as adsorption, membranes, electrocoagulation, electrodialysis, electro-oxidation, chemical oxidation, bio-concentration, etc., and biological treatments including some combinations [8,9]. Particularly, Dávila et al [10] studied a process of electrocoagulation- electroflotation in the treatment of vinasse and was achieved remove only 37% of the total solids by using aluminum anodes and was not studied the effect over phenols content.
Anaerobic digestion represents an promissory alternative because it is possible to obtain biogas from vinasse [11]; however, it has been found that phenols content leads to inhibitory effects in microorganisms used[12]. For this reason, anaerobic treatments combined with advanced oxidation pre-treatments have been tried to mitigate this inhibitory effect [13, 14].
A combined process consisting in the electro-dissolution of iron and a posterior stage of heterocoagulation on distillery vinasse was recently evaluated [14]. Were obtained reductions in turbidity of about 99%, in color 83%, and in dissolved organic carbon (DOC) 25%, with electrical power consumption below 0.3 kWh m-3. However, the performance of this novel process by operating as a pre-treatment of a biological system had not been assessed up to now.
The aim of this study was to research the performance of the coupling between the electrodissolution-heterocoagulation and the anaerobic biodegradation of vinasse by using the toxicity indicator Daphnia pulex.
2. Materials and methods
2.1. Vinasse characteristics
Vinasse samples were taken from the bottom of the mash column in a local distillery located in Cerrito, Valle del Cauca, Colombia. The distillery produces about 300,000 liter/day of ethanol. The samples were collected every two months during consecutive ten months, and the collecting and preserving procedures followed the standards in APHA et al [16]. The physiochemical characteristics of the sample were determined according to standardized methods for analyzing drinking water and wastewater [16], and the total phenol content according to Folin Ciocalteu's method [17].
2.2. Electrodissolution and heterocoagulation (EH)
An arrangement of seven carbon-steel electrodes (ASTM A36) in a monopolar configuration was used during electro-dissolution; the electrode dimensions were 9 cm long, 6 cm wide, and a gap of 5 mm. The electrode assembly had a 324 cm2 anodic area and it was immersed into the vinasse using 800 mL of the sample to 60°C; also, it was used magnetic stirring at 240 rpm. The system was galvanostatically polarized with 1.0 mA cm-2 by a power source GPS-S 3030D, and an electrical charge of 0.278 Ah L-1 was used in all tests.
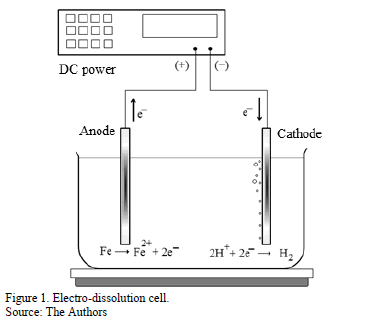
Electro-dissolution can be considered as an accelerated-corrosion process that can be achieved by keeping an anodic potential which is higher than the metal equilibrium potential. During this process, the iron cations pass to the vinasse by dissolving sacrificial anodes (Fig. 1). Different to electrocoagulation, the electro-dissolution is carried out keeping pH acid, not only to improve the dissolution kinetic, but also to avoid electrodes passivation. Besides, it is operated to low potentials to avoid the reaction of oxygen evolution in the anode.
A heterocoagulation stage, which is defined as the simultaneous coagulation of several species, was carried out to pH 11.5 using Ca(OH)2 and 150 ppm of H2O2 for oxidizing Fe2+ to Fe3+ which generates species less solubles. During this stage, a 240 rpm constant stirring was kept for 30 minutes, according to Marriaga [15]. Also, 3 ppm of anionic polyacrylamide (flopam) was added; then, the sample was strongly stirred (700 rpm) for 5 seconds. Afterwards, the stirring was stopped and a supernatant was obtained by decantation; then, a second flocculation took place by adding 500 ppm of H2O2 and 3 ppm of anionic polyacrylamide (PAM).
2.3. Anaerobic biodegradability
The used anaerobic sludge was obtained from a local effluent treatment plant; these effluents came from an abattoir. Eight 1-liter amber reactors inoculated with 2 g L-1 of volatile suspended solids (VSS) were used. Each reactor was fed with each one of the samples until they reached a concentration in COD of 3.9 gO2 L-1 of raw vinasse, and 4.5 gO2 L-1 of pre-treated vinasse by EH. 1 mL of macro and micronutrient solution was provided as well as a 0.5 mL sodium sulphide solution to 10%, and 0.15 mL of yeast extract. The pH for the operation rank between 6.5 and 7.5 units was set up.
A sludge pattern without substrate was developed at the same time to correct methane content caused by the sludge. The anaerobic biodegradability was evaluated for 30 days in terms of the COD daily removal. 3 mL of the solution were daily removed from the anaerobic reactor. The pH, the soluble COD, and methane production were measured with the volumetric method by liquid-displacement (NaOH 3%), using an experimental gasometer designed at the laboratory. The effectiveness determination of the applied treatment systems was evaluated by determining effluent toxicity using Daphnia pulex.
2.4. Toxicity evaluation with Daphnia pulex
Firstly, the rank of sensibility of the indicator organism was established before performing the bio-trial, using K2Cr2O7 as a toxic of reference. The bio-trials set-up was achieved taking a rank of 5 concentrations of each sample three times, in which less than 24-hour-old newborn/neonate were exposed. The pH was kept in a rank from 7.0 to 7.5, and the oxygen dissolved between 6.93 and 8.86 mgO2 L-1. After 48-hours exposure time, the mean lethal concentration LC50 was calculated, using the statistical software from EPA probit v1.5.
3. Results and discussion
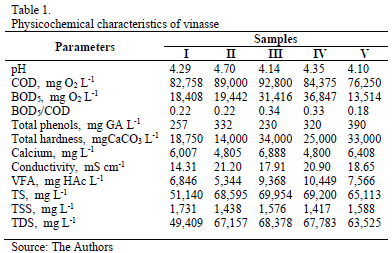
The physiochemical characteristics of the vinasse used in the tests are summarized in Table 1.
The COD and BOD5 values found indicate that vinasse is an effluent with a high organic-matter content, and susceptible to be degraded only between 18% and 34%. The high total solid content is also relevant, in which more than 97% are found as dissolved solids represented mainly as inorganic salts; besides, it presents high hardness (14,000-34,000 mg L-1) and high electrical conductivity, which diminishes power consumption during the electrodissolution.
The phenol total content (measured in milligrams of galic acid per liter) fluctuated between 230 and 390 mg GA L-1; this variation could be related to the different varieties and the ripeness degree of the sugar cane used to obtain the cane juice. In this sense, Larrahondo [18] points out that sugar cane juices are made of phenolic compounds, which can be simple or complex as flavonoids that are highly soluble in water. Vinasse composition also depends on the raw material used in the fermentation process, i.e. juice, molasses, syrup or a mixture of them; it also depends on the type and efficiency of fermentation and distillation stage as well as the varieties and the ripeness time of cane [19].
In general, it was found that the parameter values exceeded the legal discharging limits (Table 1), according to act 1594, 1984 (Ministerio de Agricultura Colombiano) e.g the discharge limit for total phenols is 0.2 mg L-1.
3.1. Electro-dissolution and hetero-coagulation (EH)
In this stage, the flocculant polymer (PAM) accomplished the function of increasing the size of the generated flocs during the hetero-coagulation. Due to that the insoluble aggregates of ferric species formed have negatives superficial charges, the calcium cations are attracted to the surface of these species and modifies the superficial charge of them to positive values. For this reason, the branched anionic polymer used can act as flocculant of this system.
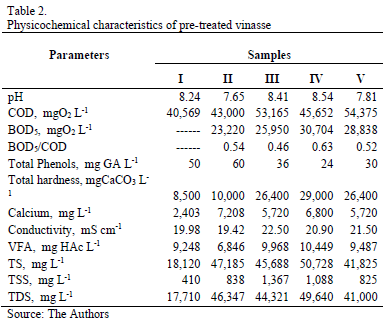
Using the EH pretreatment (Table 2), the oxidizable compounds content, which was measured as COD, was decreased between 29-52%. BOD5 behavior was variable, increasing and decreasing depending on sample. However, the general trend was the increasing in the proportion of biodegradable organic matter (BOD5/COD) from 0.18-0.24 to 0.46-0.63. Total solids (TS), total suspended solid (TSS) and total dissolved solids (TDS) were also reduced to 27-65%, 13-76% and 27-64% respectively. Likewise, the total hardness was diminished between 20% and 55%; nevertheless, the dissolved calcium content was maintained or tended to increase due to Ca(OH)2 addition during hetero-coagulation.
Finally, a slight increasing of content of volatile fatty acids (VFA) was observed, nearly to 18%; this indicates that part of the long-chain organic acids were transformed to shorter-chain ones during the EH pretreatment [20]. The VFA in vinasse is attributable to reactions due to bacterial contamination and are performed during the fermentation. In these reactions are produced organic acids mainly acetic, propionic and butyric acids.
The most remarkable result during the pretreatment was the decreasing of total-phenol content, which was between 80% and 92%. These removals are higher than those obtained by Siles et al (40%-65%) [13], and those reported by Martin et al (65%-83%) [14]. Both studies used ozone as oxidizing agent in the pretreatment and, in these cases, the initial phenol concentrations were 450-660 ppm, respectively.
Although the mechanism governing the heterocoagulation can be very complex, it is possible to realize that to pH alkaline, insoluble salts precipitation like phosphates, and calcium and iron carbonates are caused [21, 22]. It is also valid to foresee the simultaneous coagulation of ferric species [23], iron hydroxysalts [24], besides organic matter adsorption caused by the presence of Ca(OH)2 y Mg(OH)2 in suspension [25]. Therefore, the phenol-content reduction and COD could be attributed to the adsorption influence on generated insoluble species.
Also, applying hydrogen peroxide during treatment at alkaline pH generates the hydroperoxyl ion (HOO¯). The hydroperoxyl ion converts chromophore groups rich in electrons e.g carbonyl groups with conjugated phenolic, to its counterpart non-chromophore due to that is a strong nucleophile. This can explain the significant reduction of color in the pretreated vinasse.
3.2. EH and anaerobic biodegradability
Based on literature values and references [26, 27], the inoculum characterization showed that the sludge employed as a inoculum complies all requisites and minimum characteristics for the anaerobic treatment with respect to TS, VTS, stability and EMA.
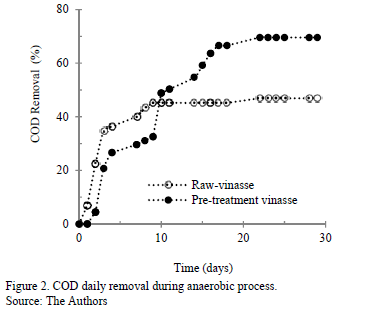
The treatment was evaluated in terms of filtered-COD content reduction. Fig. 2 shows the biodegradability behavior related to COD daily reduction. The degradation process began rapidly, which suggests that the inoculum appropriately adapted with raw and pretreated vinasse. At the beginning, the COD removal rate was higher in raw vinasse; however, from the tenth day of digesting, the COD removing rate in pretreated vinasse was much higher than the raw vinasse. At the end of treatment, COD removal rates near to 47% for raw vinasse and 70% for pretreated vinasse were obtained. This shows that the raw vinasse is more resistance to degradation than pretreated vinasse, which can be attributed to higher phenol-compound content and to a low BOD5/COD relationship [28].
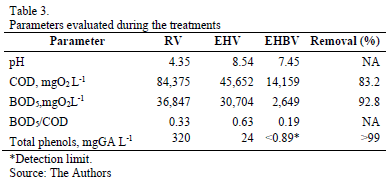
Table 3 shows the results of raw-vinasse (RV) physiochemical characterization, the pretreatment vinasse using electro-dissolution and hetero-coagulation processes (EHV), and the vinasse treated through coupling treatments (EHBV).
The most remarkable result was the 99% reduction in total phenols content, whose final concentration was below the detection limit of the used technique. On the other hand, the biodegradable organic matter content, measured as BOD5, was reduced nearly 93%, and the oxygen chemical demand was reduce to nearly 83%.
3.3. Toxicity evaluation using Daphnia pulex
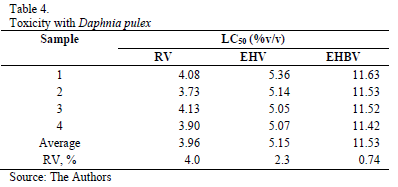
The sensitivity range of Daphnis colony compared to K2Cr2O7 was between 0.405 and 0.557 ppm on 20 analyzed samples and a standard deviation of 0.036. The variation coefficient reached an average of 7.49%, lower than the maximum variability value (RV 30%) established by the USEPA. In the tests, the pressure generated was 75% higher than expected. The results of the toxicity tests using Daphnia pulex are summarized in Table 4.
The obtained results show that raw vinasse presented the highest toxicity with median lethal concentration (LC50) of 3.96 v/v. By applying the electro-dissolution-hetero-coagulation (EHV), LC50 magnitude increased in about 30% in comparison to the results obtained in raw vinasse. This toxicity decrease would be mainly associated to phenol-content reduction.
Coupling electro-dissolution, heterocoagulation and anaerobia digestion (EHBV) LC50 magnitude increased in about 190% in comparison to the results obtained in raw vinasse. This indicates that the coupling treatment effluent presented lower toxicity than raw vinasse.
4. Conclusions
A novel treatment for vinasse was evaluated using as pretreatment a stage of electro-dissolution of iron and hetero-coagulation with Ca(OH)2 and a posterior biological treatment. During the pretreatment was obtained decreasing of phenol content of 92%, the chemical oxygen demand decreased 52%, and the volatile fatty acids content increased 18%. Afterwards, the pretreated vinasse was subjected to anaerobic digestion and was reached a global diminution close to 93% in BOD5; 83% in COD and up to 99% in phenol content. In addition, a toxicological evaluation with Daphnia pulex was carried out and it was found an increasing of 190% in the median lethal concentration (LC50) after the proposed coupling.
It were found promissory results by using iron electro-dissolution and hetero-coagulation as a pretreatment of anaerobic digestion to remove phenolic compounds and to enhance the degradation of distillery vinasse.
References
[1] Jiménez, A.M. and Borja, A., Aerobic-anaerobic biodegradatiom of beet molasses alcoholic fermentation wastewater., Process Biochem., 38, pp. 1275-1284, 2003. [ Links ]
[2] Larrahondo, J.E., Morales, A.M. y Victoria, H., Compuestos orgánicos en vinaza. Carta trimestral Cenicaña, 22 (3), pp. 5-7, 2000. [ Links ]
[3] Figaro, S., Louisy-Louis, S. and Lambert, J., Adsorption studies of recalcitrans compounds of molasses spentwash on activated carbons., Water Resources, 40, pp. 3459-3466. 2006. [ Links ]
[4] Parnaudeau, V., Condom, N., Oliver, R. and Cazevieille, P., Vinasse organic matter quality and mineralization potential, as influenced by raw material, fermentation and concentration processes., Bioresource Technology, 99, pp. 1553-1562, 2008. [ Links ]
[5] Subramanyam, R. and Mishra, I., Biodegradation of catechol (2-hidroxy phenol) bearing wastewater in an UASB reactor., Chemosphere, 69, pp. 816-824, 2007. [ Links ]
[6] Hernández, J. and Edyvean, R., Inhibition of biogas production and biodegradability by substituted phenol compounds in anaerobic sludge., J. Hazard. Mater., 16, pp. 20-28, 2008. [ Links ]
[7] Aruoja, M., Sihtmae, M. and Dubourguier, H., Toxicity of 58 substituted anilines and phenols to algae Pseudokirchneriella subcapitata and bacteria Vibrio fisheri. Comparison with published data an QSARs., Chemosphere, 84, pp. 1310-1320, 2011. [ Links ]
[8] Mohana, S., Bhavik, K., Acharya, B. and Madamwar, D., Distillery spent wash: Treatment technologies and potential applications., J. Hazard Mater., pp. 12-25, 2009. [ Links ]
[9] Satyawali, Y. and Balakrishnan, M., Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: A review., J. Environ. Manage., 86, pp. 481-497, 2008. [ Links ]
[10] Dávila, J., Marriaga, N. and Machuca, F., Remoción de sólidos totales de vinazas por electrocoagulación - electroflotación, DYNA, 76 (158), pp. 41-47, 2009. [ Links ]
[11] Pant, D. and Adholeya, A., Biological approaches for treatment of distillery wastewater: A review., Bioresource Technol., 98, pp. 2321-2334, 2007. [ Links ]
[12] Chen, Y., Cheng, J. and Creamer, K., Inhibition of anaerobic digestion process: A review., Bioresource Technol., pp. 4044-4064, 2008. [ Links ]
[13] Siles, J., García-García, I., Martín, A. and Martín, M., Integrated ozonation and biomethanization treatments of vinasse derived from ethanol manufacturing., J. Hazard. Mater., 188, pp. 247-253, 2011. [ Links ]
[14] Martín, M., Raposo, F., Borja, R. and Martín, A., Kinetic study of the anaerobic digestion of vinasse pretreated with ozone, ozone plus ultraviolet light, and ozone plus ultraviolet light in the presence of titanium dioxide., Process Biochem., 37, pp. 699-706, 2002. [ Links ]
[15] Marriaga-Cabrales, N., Uso combinado de electrodisolución, heterocoagulación y decoloración alcalina para el tratamiento de vinazas, PhD. dissertation, Escuela de ingeniería química, Universidad del Valle, Cali, Colombia, 2013. [ Links ]
[16] American Public Health Association (APHA), Standard methods for the examination of water and wastewater, Washington, D.C. 2005. [ Links ]
[17] García, I., Bonilla, J. and Jiménez, P., Biodegradation of phenol compounds in vinasse using Aspergillus terreus and Geotrichum candidum., Water Res., pp. 2005-2011, 1997. [ Links ]
[18] Larrahondo, J.E., El cultivo de la caña de azúcar en la zona azucarera de Colombia. En: Cassalett, C.. Torres, J. y Isaacs, C., Eds., Calidad de caña. Cenicaña, Cali, 1995, pp. 337-354. [ Links ]
[19] García, A. y Rojas, C., Posibilidades de uso de la vinaza en la agricultura de acuerdo con su modo de acción en los suelos., Tecnicaña, 10, pp. 3-13. 2006. [ Links ]
[20] Dinsdale, R., Hawkes, F. and Hawles, D., Anaerobic digestion of short oganics acids in an expanded granular sludge bed reactor., Water Res., 34, pp. 2433-2438, 2000. [ Links ]
[21] Voegelin, A. et al., Effect of phosphate, silicate, and Ca on Fe(III)-precipitates formed in aerated Fe(II)- and As(III)-containing water studied by X-ray absorption spectroscopy., Geochim. Cosmochim. Ac., 74, pp. 164-186, 2010. [ Links ]
[22] Scaccia, S., Carewska, M., Bartolomeo, A. and Prosini, P., Thermoanalytical investigation of iron phosphate obtained by spontaneous precipitation from aqueous solutions. Thermochimica Acta, 383, pp. 145-152, 2002. [ Links ]
[23] Hove, M., van Hille, R. and Lewis, A., Mechanisms of formation of iron precipitates from ferrous solutions at high and low pH., Chem. Eng. Sci., 63, pp. 1626-1635, 2008. [ Links ]
[24] Kok-Hui, G., Teik-Thye, L. and Zhili, D., Application of layered double hydroxides for removal of oxyanions: A review., Water Res., 42, pp. 1343-1368, 2008. [ Links ]
[25] Semerjian, L. and Ayoub, G., High-pH-magnesium coagulation-flocculation in wastewater Treatment., Adv. Environ. Res., 7, pp. 389-403, 2003. [ Links ]
[26] Field, J., Medición de parámetros. En: Manual de arranque y operación de sistemas de flujo ascendente con manto de lodo - UASB., Cali: Universidad del Valle, CVC, Universidad agrícola de Wageningen, 1995. [ Links ]
[27] Espitia, S., Vargas, C. y Díaz-Báez, M., Caracterización fisicoquímica y microbiológica de lodos utilizados en plantas de tratamiento anaerobio., s.l.: Universidad Católica de Valparaíso, 1998. [ Links ]
[28] Fitzgibbon, F., Sligh, D. and McMullan, G., The effect of phenolic acids and molasses spent wash concentration on distillery wasterwater remediation by fungi., Process Biochem., 27, pp. 1527-1534, 1998. [ Links ]
O. L. Paz-Pino, received the Bs. Chemistry in 2013 from the Universidad del Valle. Cali, Colombia. Since 2013 until now he works as Full Teacher in a local school.
L. E. Barba-Ho, received the Bs.in Chemistry in 1976, the MSc. in Chemical Sciences-Chemistry in 1983, all them from Universidad del Valle, Cali-Colombia. From 1983 to 1988, she worked at the Corporacion Autonoma Regional del Valle del Cauca - CVC, as director of the Environmental Laboratory and as superviser of different Environmental projects. From 1988 until now, she has been a full time proffesor in the subjects of Environmental Chemistry and Environmental Physical Chemistry at the Universidad del Valle, Cali, Colombia. Her research's field is the toxic compounds as pesticides and heavy metals, toxicity assays in the wastewater treatment and solid residue and the couple physical-biologycal system. She is too the director of the environmental Laboratory acredited with NTC-ISO/IEC 17025 de 2005.
N. Marriaga-Cabrales, received the Bs. Eng in Chemical Engineering in 1997, the MSc. degree in Chemical Engineering in 2001, both from the Universidad Industrial de Santander. Bucaramanga, Colombia. He got the PhD. degree in Engineering in 2013, from the Universidad del Valle. Cali, Colombia. Since 2002 until now he works for the Universidad del Valle Colombia. Currently, he is a Full Professor in the Chemical Engineering School, Engineering Faculty, Universidad del Valle, Colombia. His research interests include: Electrochemical techniques to treat wastewater as electrocoagulation, and electro-oxidation, advanced oxidation processes, and chemical process design.