Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.187 Medellín set-/out. 2014
https://doi.org/10.15446/dyna.v81n186.40758
http://dx.doi.org/10.15446/dyna.v81n187.40758
Electrodes friendly with the environment for detect heavy metal
Electrodos amistosos con el medio ambiente para detectar metales pesados
Jairo Alberto Barón-Jaimez a, José Luddey Marulanda-Arévalo b & José José Barba-Ortega c.
a Universidad Nacional Autónoma de México, México.
b Grupo GIMAV, Universidad Tecnológica de Pereira, Colombia. jlmarulanda@utp.edu.co
c Departamento de Física, Universidad Nacional de Colombia, Bogotá, Colombia. jjbarbao@unal.edu.co
Received: November 9th, 2013. Received in revised form: June 3th, 2014. Accepted: June 9th, 2014.
Abstract
In the last centuries, heavy metals have been extracted and processed industrially; today, they are part of our food chain and their detection has generated great international interest due to adverse effects, beyond allowed amounts for humans. For many years, mercury electrodes have been used to detect heavy metals; however, due to the new regulations and its high toxicity, new alternatives for its replacement as electrode in electroanalytical techniques have been investigated. Bismuth electrodes have been positioned as an alternative to this type of activity because of its many features similar to mercury and low toxicity. This paper provides an overview of this new type of electrode, along with different parameters to consider in order to obtain better results
Keywords: Bismuth, Electrodes, Voltammetry, Heavy Metals.
Resumen
Los últimos siglos, los metales pesados han sido extraídos y procesados industrialmente; hoy en día, hacen parte de nuestra cadena alimenticia y su detección ha generado gran interés a nivel internacional debido a los efectos adversos, en cantidades más allá de las permitidas para el ser humano. Por muchos años, los electrodos de mercurio han sido utilizados para detectar metales pesados; sin embargo, debido a las nuevas regulaciones y su alta toxicidad, nuevas alternativas para su reemplazo como electrodo en las técnicas electroanalíticas han sido investigadas. Los electrodos de Bismuto se han situado como una alternativa para este tipo de actividad debido a sus múltiples características semejantes a las del mercurio y su baja toxicidad, en este documento se presenta un panorama de este nuevo tipo de electrodo, junto con diferentes parámetros a tener en cuenta para obtener mejores resultados.
Palabras clave: Bismuto, Electrodos, Voltamperometría, Metales Pesados
1. Introduction
Heavy metals are natural components of the Earth's crust. They cannot be degraded or destroyed. Their trace elements are considered to be one of the main sources of pollution in the environment. To a small extent they enter our bodies via food, drinking water and air. As trace elements, some heavy metals (e.g. copper, zinc) are essential to maintain the metabolism of the human body; However, even at low concentrations some of them can be toxic, for this reason they are recognized as highly toxic and dangerous contaminants, only pesticides outweigh their danger and toxicity [1,2,3,4].
Increasing industrialization has been accompanied throughout the world by the extraction and distribution of mineral substances from their natural deposits. Many of these have undergone chemical changes through technical processes and finally, they stay finely dispersed through effluent into water, earth, air and thus into the food chain, It is unlikely that they can be removed, by washing (e.g. lead and cadmium contamination). Besides, the contamination by fertilizers and insecticides is becoming an increasing problem, their widespread use results in their frequent appearance in the environment and foods [2,4,5]. The importance of controlling the levels of these pollutants in natural waters, drinking water, sediments and industrial waste has become an international political topic, so it has generated great interest in the development of new analytical methodologies for pollution level determination [2,6].
Different techniques and methods have been developed for trace metals determination, atomic absorption spectrometry (AAS), electrothermal atomic absorption spectrometry (ETAAS), Neutron activation techniques, flame atomic absorption spectrometry (FAAS), inductively coupled plasma atomic emission spectrometry (ICP-AES), inductively coupled plasma mass spectrometry (ICP-MS), and inductively coupled plasma optical emission spectrometry (ICP-OES), but they are not used frequently because of the specialized techniques, long time, high cost, and are unsuitable for applications in the field. In addition, the analysis must be performed in a specialized laboratory by skilled personnel [1,4,6,7]. Electrochemical stripping analysis has long been recognized as a powerful technique for trace metals owing to its remarkable sensitivity, relatively inexpensive instrumentation, ability for multi-element determination and capacity to determine elements accurately at trace and ultra-trace levels [4], and is beginning to be considered the most sensitive electro-analytical technique for the determination of trace metals in samples of environmental, clinical and industrial origin [8,9,10]. Its extraordinary sensitivity is attributed to its effective step of pre-concentration "this step generates a best sensitivity, with an increase of 2 or 3 orders of magnitude" together with advances in electrochemical measurements of accumulated analyte [3,11,12]. In this review, we provide an overview of different works related to Bi electrodes and their applications for the detection of heavy metals and other substances in environmental, food and clinical samples.
2. Stripping voltammetry
Among of different voltammetric techniques, stripping voltammetry (SV) has been receiving considerable attention since it is the most sensitive electroanalytical technique, mgL-1 (ppb) and even ngL-1 (ppt) detection limits have been reported, and over the last decade it has evolved into a very versatile and powerful analytical technique. This electrochemical method encompasses a variety of electroanalytical procedures having a common characteristic initial step. In all these procedures; the analyte of interest is accumulated on a working electrode by controlled potential electrolysis, while the solution is stirred. After a short rest period (a few seconds), this preconcentration step is followed by the stripping step, which involves the dissolution of the deposit when a potential sweep is applied to the electrode. Thus, a detectable current is produced at the electrode surface following the oxidation or reduction of the analyte at a characteristic potential [13-16].
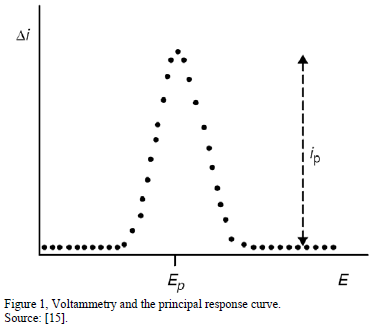
By careful interpretation of the resulted peak shape from current-potential voltammogram recorded during the stripping step, important and desired analytical information is readily obtained. The peak potential (position of Ep) is characteristic of the given substance and thus it can be used for qualitative identification, whereas the peak current Ip is proportional to the concentration of the corresponding analyte in the test solution. This analytical quantitative information can be obtained from the height or area of the stripping voltammetric peak. Since stripping curves/peaks for various analytes occur at characteristic potentials, hence several species can often be determined simultaneously. [3,14-16].
3. Potential window
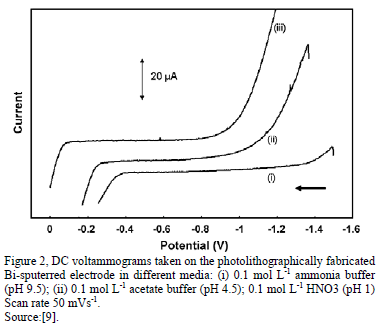
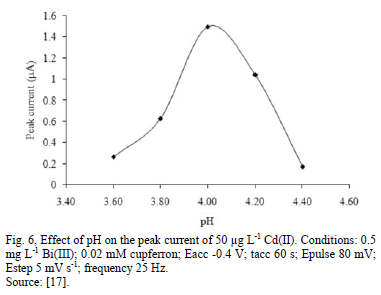
All voltammograms of Bismuth displayed a wide flat middle potential region called "potential window", which is ubicated between -1.2 to -0.2 V vs. SCE (Saturated Calomel Electrodes) approximately [11]. This potential window can be moved to more negative or positive potentials in accordance with the pH; for high pH, the potential window is displaced to more negative potentials and for low pH the contrary. However, it should be contemplated that for more negative potentials (cathodic side) the reduction of hydrogen ions caused an increase in the background current. This also happened at more positive potentials (anodic side) caused by the oxidation of bismuth [9]. In some studies, it has been described that the peak current is affected by the pH, for example, the peak current of cadmium was at maximum value, the reduction of peak current were decreasing both at lower and higher pH values due to the protonation of coordination sites of the ligand and the hydrolysis of the Cd(II), respectively [17].
Generally, for the determination of Cd(II) and Pb(II), strongly acidic media are avoided to prevent excessive hydrogen evolution that could interfere with the deposition process and alkaline media are avoided because of the limited anodic range that would cause difficulties in the determination of Pb (since in alkaline media bismuth oxidizes at a more negative potential) [9].
4. Bismuth electrodes
Since the invention of polarography by Heyrovsky [3,18]; Mercury in the form of the hanging mercury drop electrode (HMDE) or the mercury-film electrode (MFE) has been the most common electrode material in electroanalysis [8-11,18-21]. The advantages of mercury based electrodes are numerous, such as the high hydrogen overpotential allowing its use at useful negative potentials, the sensitivity and reproducibility; however, because of the toxicity of mercury, and recent and future regulations, occupational health considerations may severely restrict the use of mercury. It is important to develop environmental friendly electrodes for stripping voltammetric determination of heavy metals [9-12, 16-23].
Metals such as Au, Ag, Sb, Ir, W as well as bare and coated carbon electrodes have all been investigated for stripping analysis of metals but none of these materials was found to be an acceptable replacement for common mercury electrodes [10,11,18]. In 2000, a new type of electrode, the BiFEs (Bismuth Film Electrodes), was proposed as an alternative to MFEs (Mercury Film Electrodes) [8,9,18,19,23]. In particular, its inherent low toxicity, the property of bismuth to form "fused alloys" with heavy metals at low temperature, facilitating the nucleation process during accumulation of heavy metal ions, insensitivity to dissolved oxygen (essential characteristic for onsite Monitoring), relatively large applicable negative potential window, well-defined and undistorted stripping signal, excellent resolution of neighboring peaks together with high sensitivity and simple preparation suggest wider practical application of bismuth-based electrodes [10-12,19-21,23,24]. Bismuth film electrodes (BiFEs) consisting of a "film" of bismuth on a suitable substrate, among the various substrate materials for BiFEs, are gold, platinum, silicon, glassy carbon, carbon fiber, bored doped diamond, screen printed carbon ink, wax impregnated graphite, pencil-lead, carbon paste electrode (CPE), the choice is determined by costs, commercial availability, easy preparation, renewable surface and stability in various solvents [3,8,12,13,17,22,26]. These electrodes are applicable for measurements of electroplated elements with standard potentials more negative than bismuth. In some instances, it may be possible to apply the bismuth electrode for measuring metals with more positive potentials. One example involves trace measurements of copper [10].
Research has been focused on the measurement of trace lead, cadmium, and zinc, copper, indium, tin, thallium, nickel, uranium, aluminum and cobalt, in some cases assessment of the formation and prevention of intermetallic compounds, showing new insights into the performance of bismuth-coated electrodes [10,11].
Different parameters should be considered in ASV with this kind of electrodes, among others:
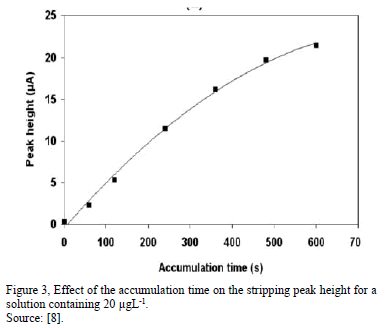
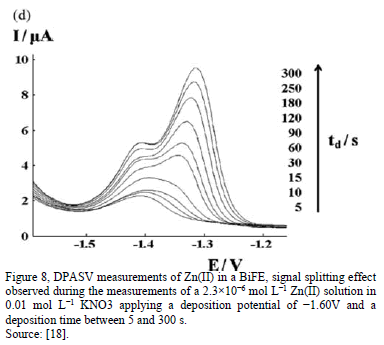
The deposition time and its effect, is an important factor on the stripping peak heights of different elements to be detected, the responses of the metals increases "lineally" with increasing the deposition time in a first zone. When the deposition time exceed the optimum time, the peak currents became almost constant "second zone", indicating that the amount of element to detect on the electrode surface achieves saturation [20,21,23].
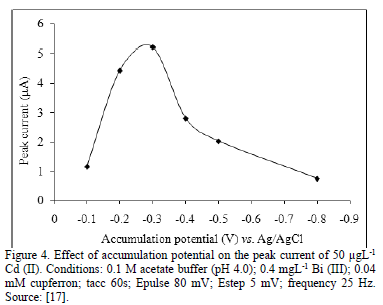
Accumulation potential is another important parameter that influences the sensitivity of the determination of element in stripping techniques, when accumulation potentials shift to more negative potential than the optimum; the peak currents became poor, because hydrogen reduction is beginning to be significant at such negative potentials. The hydrogen bubbles might damage the metal alloys deposited on the electrode surface and lead to decrease in current signals at very negative potentials. In addition, some other chemicals may be reduced, which can affect the selectivity of the electrode and interfere in the determination. For potentials more positive than the optimum potential, the background current became higher [12,17,21,23].
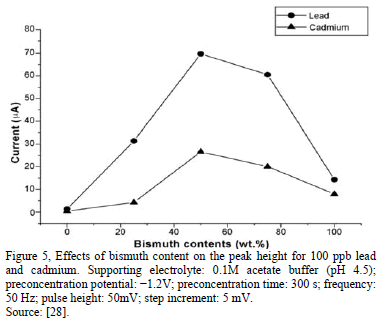
When stripping voltammetry is carried out in situ, it is important to know the effect of the bismuth concentration, although the thickness of the film not affected by the peak position of any metals, at lower concentrations that the optimum concentration of Bi, there is not sufficient to form a multicomponent of Bi with the metals; in contrast, when the Bi concentration is higher, bismuth will hold back the target metals, and affect the peak currents of metals [21,23,27,28].
The pH affects the peak current [17,20,21], the peak current decreases both at lower and higher than optimum pH values due to the protonation of coordination sites of the ligand and the hydrolysis to the Cd(II) [17].
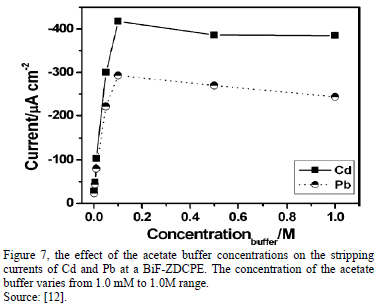
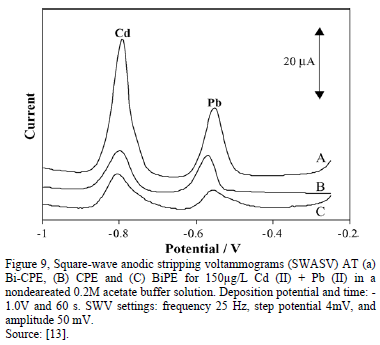
The effect of the concentrations of the acetate buffer was studied too, "BiF-ZDCPE electrodes". The results show that the stripping currents for Cd and Pb increased rapidly with the increase of the buffer concentrations from 1.0mM to 0.10 M. At high concentration of acetate buffer (>0.10 M), the stripping signals of both Cd and Pb decreased slightly. This indicates that the metal-Bi alloy formation under the applied conditions strongly depends on the ionic strength of the solution, clearly [12]. In most of the work, the form of current peak is isometric, evidencing a unique peak; however, in some case, the voltammgrams can produce two overlapping peaks, the reasons for such peak splitting are unclear and to know which peak will predominant, too (first or second signal); however, it should be related with the deposition step, one possible explanation is that two layers (or two different association mechanisms) are present in the electrode [3,19].
The formation of intermetallic compounds is considered a serious interference in the determination of some heavy metals by ASV on BiFEs. The well-known interference of Cu (II) in the determination of Pb and Cd has been reported previously on electroplated BiFEs, both cadmium and lead do not compete with the bismuth for the surface site; the stripping behavior of copper at the bismuth coated electrode is different and this affects the current peak of Bi. The interference of Cu (II) has been attributed both to the formation of mixed compounds "Example Cu-Zn" and to the undesired deposition of Cu instead of Bi. Some works show how this interference can be alleviated by the addition of element different (Ga, ferrocyanide) in the sample solution [9,10,27].
5. Modified Bi electrodes
Several methods for the generation of the bismuth film have been reported including modified electrode of bismuth powder, bismuth precursor compounds (such as Bi2O3 or BiPO4), disposable bismuth-coated porous screen-printed carbon electrode (Bi-P-SPCE), bismuth-enriched alloys and ultrasound-facilitated bismuth film formation. Bismuth-powder modified with carbon paste results in a convenient and reliable electrochemical sensor for trace heavy metal detection in conjunction with stripping electroanalysis, which has favorable properties with respect to other bismuth-based electrodes. The most common methods for the generation involves in situ or ex situ electrochemical plating by reduction of Bi (III) ions to metallic bismuth on a suitable supporting material [13,22, 28-30].
Different kinds of modified Bi electrodes have been studied to improve the electroanalytical characteristics in accordance with the mechanical and electrochemical properties of the new component. Polymeric membranes can protect the bismuth surface against abrasion, adsorption of surface-active compounds and formation of intermetallic compounds; offering a well-defined stripping peak with favorable signal-to-background characteristics, not prone to oxygen interference and enhance the sensitivity of the bismuth film for determining heavy metals. Modified Bismuth electrodes with membranes such as overoxidized poly-1-naphtylamine, poly (sodium 4-styrenesulfonate), bismuth/poly (aniline) and poly (paminobenzene sulfonic acid) (poly(p-ABSA)) can avoid the interference and stabilize the thin film at the substrate surface by creating a framework for mechanical support and strong adherence to the electrode surface, this is very attractive for practical stripping applications, but by far the most widespread studied coating films are composed of Nafion. Nafion is chemically and thermally inert, non-electroactive, conductive and insoluble in water and is, therefore, particularly suitable for the modification of electrodes. In addition to its protective properties, Nafion acts as an ion-exchanger facilitating the preconcentration of cationic target analytes, helps to mechanically stabilize the underlying bismuth layer and, under certain circumstances, can improve the detection sensitivity [21,23,27,31].
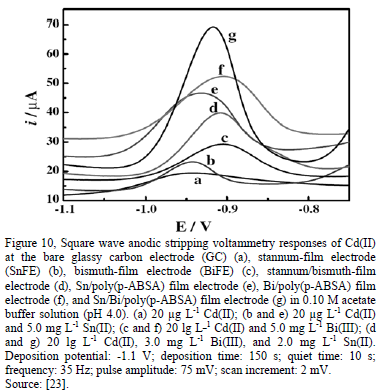
The sensitivity of the modified electrodes can improve significantly with the incorporation of metallic stannum [23]. In recent years, the antimony-film electrode (SbFE) has been reported to perform similar to the BiFE in ASV; this newly proposed electrode offers a remarkable performance in more acidic solutions (pH≤2), which can be advantageous in electrochemical analysis of trace heavy metals [20].
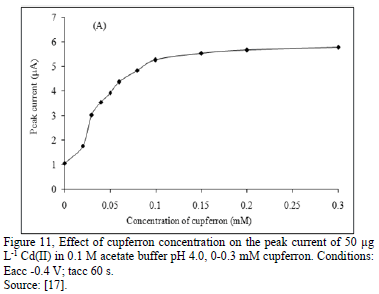
Some works show how the addition of an analytical reagent in a small amount, can enhance the current peak in the determination of some heavy metals. Cupferron in the range 0.0 to 0.1 Mm improve electrochemical signal of Cd (II) about two times. This enhancement of the electrochemical signal for Cd (II) can be improved when the concentration of Nafion increases in small percentage [17].
Other material have been added to the Bismuth electrodes such as Zeolite, the increase of zeolite content up to 5% in the carbon paste improves the stripping currents of Cd, Pb, and Bi. This can be attributed to the good ion exchange properties of zeolite. However, when the content of zeolite in the carbon paste was higher than 5%, all the peak currents of the metals decreased. The reasons may be attributed to zeolite being an insulator [12].
6. Important of the research
Heavy metals have become a focus of public interest since analytical techniques have made it possible to detect them even in very small traces. Lead has been mined since ancient times and has been processed in many ways. Cadmium exists in low concentrations in all soils and today is regarded as the most serious contaminant of the modern age, because is absorbed by many plants, sea creatures and, because of its toxicity, it presents a major problem for foodstuffs. Similarly the contamination by fertilizers and insecticides become an increasing problem, the widespread use result in their frequent appearance in the environment and foods, such as nitroguianidine neonicotinoid [2,5].
Bismuth electrodes have been applied to detect heavy metals in different samples such as biological sample (hair, urine, sweat, saliva [20] and blood [3], environment samples (air [3], tap water [3,12,13, 19-21, 23] sewage water [3,12, 13] river water [3,9,21] lake water [31], soils [3,20], foods (Cabbage, Lettuce, Celery, spinach [27], tea, wine and tomato sauce[3]), marine algae [17], plant extracts [20] and gasoline[3]. Other interesting applications of Bismuth Electrodes have begun to draw attention such as the determination of daunomycin, an effective drug used for cancer chemotherapy [11]. For the last thirteen years, studies of different Bi and modified Bi electrodes have shown that they can be considered an important environmental opportunity to voltammetry. Lead and cadmium are some of the metals most referenced in these works with Bismuth electrodes, maximum safe limits defined by the World Health Organization (WHO) for Lead are 10 mg/L for drinking water, 0.3 mg/g for vegetables and 0.2 mg/g for maize and bean. Similarly, for Cadmium, the maximum content recommended in tap water is 3.0 mg/L [20,32,33], these values can be detected according to the detection limits presented in the different investigations. Bi electrodes are emerging as one of the best potential replacements for mercury electrodes in order to implement the new stringent regulations for heavy metals (Hg, Cadmium, Lead, etc.) due to their multiple electroanalytical characteristics.
References
[1] Suwannasom, P. and Ruangviriyachai, C., Simultaneous quantification of Cd, Cu, Pb and Zn in Thai fermented food by DPASV with a microwave digestion, International Food Research Journal, 18, pp. 803-808, 2011. [ Links ]
[2] Auroville, Innovative Urban Management IND-015, Heavy metals and pesticides residue in the foodstuff, Final Report, Annexes, 2003, pp. 1-9. [ Links ]
[3] Barón-Jaimez J.A., Electrodos de Bismuto para Detectar Metales Pesados, MSc. Tesis, Universidad Nacional Autónoma de México UNAM, México, 2012. [ Links ]
[4] He, X., Liping, Z., Dekun, H., Yuezhong, X. and Litong, J., A Nafion-coated bismuth film electrode for the determination of heavy metals in vegetable using differential pulse anodic stripping voltammetry: An alternative to mercury-based electrodes, Food Chemistry, 109, pp. 834-839, 2008. [ Links ]
[5] Guzsvány, V., Papp, Z., Zbiljić, J., Vajdle, O. and Rodić, M., Bismuth modified Carbon-Based electrodes for the determination of selected neonicotinoid insecticides, Molecules, 16, pp. 4451-4466, 2011. [ Links ]
[6] IFCS, Metales pesados: ¿Necesidad de más acciones globales?, 5ta Reunión del Foro Interguvernamental de Seguridad Química, Budapest, Hungría, pp. 1-16, 2006. [ Links ]
[7] Estévez-Hernández, O.L., Furoiltioureas: Naturaleza de sus complejos con CdCl2 y HgCl2 y su utilización en sensores electroquímicos, Tesis, Universidad de Cádiz, España, 2006. [ Links ]
[8] Kokkinos, C., Raptis, L. and Economou, A., Disposable micro-fabricated electrochemical bismuth sensors for the determination of Tl(I) by stripping voltammetry Speliotis T., Procedia Chemistry, I, pp. 1039 -1042, 2009. [ Links ]
[9] Kokkinos, C., Economou, A., Raptis, I. and Efstathioua, C., Lithographically fabricated disposable bismuth-film electrodes for the trace determination of Pb(II) and Cd(II) by anodic stripping voltammetry, Electrochimica Acta, pp. 5294-5299, 2008. [ Links ]
[10] Wang, J., Lu, J., Kirgöz, U., Hocevar, S. and Ogorevc, B., Insights into the anodic stripping voltammetric behavior of bismuth film electrodes, Analytical chimical Acta, 434 pp. 29-34, 2001. [ Links ]
[11] Duwensee, H., Adamovski, M. and Flechsig, G.U., Adsorptive stripping voltammetric detection of daunomycin at mercury and bismuth alloy electrodes, International Journal of Electrochemical Science, 2, pp. 498-507, 2007. [ Links ]
[12] Caoa, L., Jia, J. and Wang, Z., Sensitive determination of Cd and Pb by differential pulse stripping voltammetry with in situ bismuth-modified zeolite doped carbon paste electrodes, Electrochimica Acta, 53, pp. 2177-2182, 2008. [ Links ]
[13] Hocevar, S., Svancara, I., Vytras, K. and Ogorevc, B., Novel electrode for electrochemical stripping analysis based on carbon paste modified with bismuth powder, Electrochimica Acta, 51, pp. 706-710, 2005. [ Links ]
[14] Alghamdi, A.H., Applications of stripping voltammetric techniques in food analysis, Arabian Journal of Chemistry, 3, pp. 1-7, 2010. [ Links ]
[15] Cornelis, R., Crews, H., Caruso, J. and Heumann, K., Handbook of elemental speciation: Techniques and methodology, Chapter 5.9 Speciation Analysis by Electrochemical Methods, 2003. [ Links ]
[16] Baron, J., Silva-Bermudez, P. and Rodil, S.E., Sputtered Bismuth thin films as trace metal electrochemical sensors. Materials Research Society. Symp. Proc., Vol. 1477, 2012. [ Links ]
[17] Meepun, N., Siriket, S. and, Dejmanee, S., Adsorptive stripping voltammetry for determination of Cadmium in the presence of Cupferron on a Nafion-coated Bismuth film electrode, International Journal of electrochemical Science, 7, pp. 10582-10591, 2012. [ Links ]
[18] Serrano, N., Alberich, A., Díaz-Cruz, J.M., Ariño, C. and Esteban, M., Signal splitting in the stripping analysis of heavy metals using bismuth film electrodes: Influence of concentration range and deposition parameters, Electrochimica Acta, 53, pp. 6616-6622, 2008. [ Links ]
[19] Prior, C., Lenehan, C. and Stewart-Walker, G., Utilising gallium for enhanced electrochemical copper analysis at the bismuth film electrode, Analytical Chimica Acta, 598, pp. 65-73, 2007. [ Links ]
[20] Jiao, Y.W., Li, Y., Ran, G., Qun, L.H. and Bing, L.N., Determination of cadmium (II) by square wave anodic stripping voltammetry using bismuth-antimony film electrode, Sensor and Actuators B, 166-167, pp. 544-548, 2012. [ Links ]
[21] Wu, Y., Bing, L.N. and Qun, L.H., Simultaneous measurement of Pb, Cd and Zn using differential pulse anodic stripping voltammetry at a bismuth / poly (p-aminobenzene sulfonic acid) film electrode, Sensor and Actuators B, 133, pp. 677-681, 2008. [ Links ]
[22] Kokkinos, C., Economou, A, Raptis, I., Efstathiou, C. and Speliotis, T., Novel disposable bismuth-sputtered electrodes for the determination of trace metals by stripping voltammetry, Electrochemistry communications, 9 (12), pp. 2795-2800, 2007. [ Links ]
[23] Xiong, Ch.H., Luo, H.Q. and, Li, B.N., A stannum/bismuth/poly(p-aminobenzene sulfonic acid) film electrode for measurement of Cd (II) using square wave anodic stripping voltammetry, Journal of Electroanalytical Chemistry, 651 (1), pp. 19-23, 2011. [ Links ]
[24] Bedoya, C.M., Pinzón, M.J., Alfonso, J.E., Restrepo, E. and Olaya, J.J., Physical-Chemical Properties of Bismuth and Bismuth Oxides: Synthesis, Characterization and Applications, DYNA, 79 (176), pp. 139-148, 2012. [ Links ]
[25] Kokkinos, C., Raptis, I., Economou, A. and Speliotis, T., Disposable micro-fabricated electrochemical bismuth sensor for the determination of Tlv(I) by stripping voltammetr, Procedia Chemistry I, pp. 1039-1042, 2009. [ Links ]
[26] Economou, A., Bismuth-film electrodes: Recent developments and potentialities for electroanalysis, TrAC Trends in Analytical Chemistry, 24 (4), pp. 334-340, 2005. [ Links ]
[27] Xu, H., Zeng, L., Huang, D., Xian, Y. and Jin, L. A Nafion-coated bismuth film electrode for the determination of heavy metals in vegetable using differential pulse anodic stripping voltammetry: An alternative to mercury-based electrodes, Food Chemistry, 109, pp. 834-839, 2008. [ Links ]
[28] Hwang, G.H., Han, W.K., Hong, S.J., Park, J.S. and Kang, S.G., Determination of trace amounts of lead and cadmium using a bismuth/glassy carbon composite electrode, Talanta, 77, pp. 1432-1436, 2009. [ Links ]
[29] Kadara, R. and Tothill, I., Development of disposable bulk-modified screen-printed electrode based on bismuth oxide for stripping chronopotentiometric analysis of lead (II) and cadmium (II) in soil and water samples, Analytica Chimica Acta, 623, pp. 76-81, 2008. [ Links ]
[30] Chen, C., Niu, X., Chai, Y., Zhao, H. and Lan, M., Bismuth-based porous screen-printed carbon electrode with enhanced sensitivity for trace heavy metal detection by stripping voltammetry, Sensors and Actuators B, 178, pp. 339- 342, 2013. [ Links ]
[31] Kokkinos, C., Economou, A., Disposable Nafion-modified micro-fabricated bismuth-film sensors for voltammetric stripping analysis of trace metals in the presence of surfactants, Talanta 84 (3), pp. 696-701, 2011. [ Links ]
[32] European Commission, Lead Standard in Drinking Water. 11th plenary, of 11 January 2011. [ Links ]
[33] Makokha, A., Mghweno, L., Magoha, H., Nakajugo, A. and Wekesa, J., Environmental lead pollution and contamination in food around Lake Victoria, Kisumu, Kenya, African Journal of Environmental Science and Technology, 2 (10), pp. 349-353,2008. [ Links ]
J. A. Barón-Jaimez, received the Bs. Eng. in Metallurgical Engineering in 2000 from the Universidad Industrial de Santander, Colomba. In 2012, he obtained a MSc. in Science and Materials Engineering from the Universidad Nacional Autonoma de Mexico, Mexico. Currently, he is a failure analysis Engineer in the oil and gas area. His research interests include detection of heavy metals in solutions and foods using electrochemical techniques.
J. L. Marulanda-Arévalo, received the Bs. Eng. in Metallurgical Engineering in 1999 and an MSc. in Metallurgical Engineering in 2002 from the Universidad Industrial de Santander, Colombia. In 2012 obtained his PhD. degree in Advanced Chemistry from the Complutense University of Madrid, Spain. Since 2006, he works as a professor at the Universidad Tecnológica de Pereira in the Faculty of Mechanical Engineering. His research interests include corrosion, welding, coatings, tribology and electrochemistry.
J. J. Barba-Ortega, received a BSc. In Physics in 2000 and an MSc. in Physics in 2003, from the Universidad Industrial de Santander, Colombia. He received his PhD degree in Physics in 2007, and from 2007 to 2009, he obtained Post-doctoral experience from the Universidade Federal de Pernambuco, Recife, Brasil. Currently, he is a Full Professor in the Physics Department in the Universidad Nacional de Colombia, Bogotá. His research interests include computational simulations in superconducting mesoscopics and low dimension semiconducting systems including numerical methods.