Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.81 no.187 Medellín set-/out. 2014
https://doi.org/10.15446/dyna.v81n186.40960
http://dx.doi.org/10.15446/dyna.v81n187.40960
Effect of an electrolyte flow on electrochemical hydrogen permeation
Efecto de un flujo de electrolito en la permeación electroquímica de hidrógeno
Ana María Pérez-Ceballos a, Jorge Andrés Calderón-Gutierrez b & Oscar Rosa-Mattos c
a Grupo CIDEMAT, Grupo GIPIMME, Facultad de Ingeniería, Universidad de Antioquia, Colombia. ampc@udea.edu.co
b Grupo CIDEMAT, Facultad de Ingeniería, Universidad de Antioquia, Colombia, jacalder@udea.edu.co
c Laboratorio de Ensaios Não Destrutivos, Corrosão e Soldagem -LNDC-, Universidade Federal de Rio de Janeiro, Brazil, omattos@metalmat.ufrj.br
Received: November 22th, de 2013. Received in revised form: June 10th, 2014. Accepted: July 10th, 2014
Abstract
The influence of an electrolyte flux injection over the detection side on pure iron membranes on electrochemical hydrogen permeation test was studied. A modified Devanathan's cell was used in the experiments. This cell allows the injection of an electrolyte flux over the hydrogen exit side during the permeation tests. In the hydrogen generation compartment a NaOH 0.1M + 2mgL-1 As2O3 solution was used applying a constant current of 2.85 mA. The detection side was maintained under potentiostatic control at potential values in the passivity range for iron. The solution used in the exit compartment was a borate buffer (pH=8.4). It was not observed a significant variation of the permeation current when an electrolyte flow was injected on iron samples, however, a slightly raise both of the steady state of permeation current density and the calculated permeation parameters was observed in the tests in which an electrolyte flux was applied.
Keywords: Electrochemical hydrogen permeation; pure iron.
Resumen
Se ha estudiado el efecto de la aplicación de un flujo de electrolito sobre la superficie de detección de una membrana de hierro puro en ensayos de permeación electroquímica de hidrógeno. Para la realización de los ensayos se usó una celda del tipo Devanathan modificada, de tal manera que un flujo de electrolito fue inyectado directamente sobre la superficie de salida de hidrógeno. La generación de hidrógeno se realizó aplicando una corriente catódica de 2,85mA y se usó una solución de NaOH 0,1M + 2mgL-1 As2O3. La celda de detección fue mantenida bajo control potenciostático y se usó una solución buffer de borato de sodio (pH=8,4). No se observó una variación significativa de la corriente de permeación en los ensayos realizados aplicando flujo de electrolito.
Palabras clave: Permeación electroquímica de hidrógeno; hierro puro.
1. Introduction
The interaction of hydrogen with metals has been widely studied by electrochemical permeation tests due to their simplicity and flexibility [1-8]. These measurements, generally, involve the use of the electrochemical cell developed by Devanathan and Stachurski [9]. The technique is based on a hydrogen concentration gradient created in a metallic sample to achieve hydrogen diffusion through the metal thickness. The cell is a double compartment type, the sample is a flat sheet and it is located between the two compartments, on one side of the sample hydrogen is generated, adsorbed, absorbed and diffused through the metal membrane by cathodic polarization (generation compartment). The opposite side is maintained over potentiostatic control in order to oxide the emergent hydrogen (detection compartment).
The measured permeation current is expected to be proportional to the hydrogen flux. However, the relatively poor reproducibility of the technique may conducted to misinterpretation of the results obtained [3]. Additionally, electrochemical hydrogen permeation on iron and its alloys depends on the surface cleaning, sample preparation, defects and the surface oxidation state [10-13]. The exit side surface state has a higher influence on the hydrogen permeation [14]. Furthermore, the models used to calculate permeation parameters like apparent diffusion coefficient and quantity of emergent hydrogen are proposed assuming well-defined boundary conditions. It is assumed that the hydrogen concentration at the entrance side is imposed instantaneously by both potentiostatic and galvanostatic charging. Furthermore, in all models it is assumed that the hydrogen concentration at the exit side is zero [3, 15-19]. However, some works has been demonstrated that the boundary conditions assumed are not respected during the entire permeation time [14, 20-23]. It is not a linear relationship between hydrogen steady state flow and the inverse of the membrane thickness when the hydrogen charge was made by cathodic way [24-26]. The calculation of the permeation parameters is made considering the stability of the iron surface, any interaction between hydrogen and both surfaces (in and out) is not considered [14,22,23].
Hydrogen diffusion in the metal/oxide system has been treated in similar way to the diffusion in laminated layers [27]. Due to the ionic character of oxides, hydrogen in an oxide layer must be considered as to be present in a charged species and very low hydrogen diffusion coefficients are expected in them. It is well know that the oxide coatings can act as barriers against the hydrogen entry into the metals, depending on time and on the electrochemical conditions of the surface [14, 24, 25].
The hydrogen transport through the passivated specimen could be retarded by the oxide films, mainly in the case of iron. The evolution of the passive layer avoids the stabilization of the hydrogen concentration on the detection side and induces a diminution of the hydrogen flux. Considering that the passive layer is impervious to hydrogen, the hydrogen concentration on the exit side increases, inducing lower permeation rates. That is to say, the transport of hydrogen through passivated iron is controlled mainly by the oxide [14,20,21]. Furthermore, the interaction between the hydrogen permeated at the outside of the metallic membrane and the iron oxide is not completely understood. The present study aims to investigate the interaction between the hydrogen permeated and the iron oxide presented at the detection side of the iron membrane by the application of an impinging jet of electrolyte over the detection side. The perturbation generated by a controlled impinging jet of electrolyte on the iron oxide layer could cause significant changes in the iron oxide layer and to induce changes in the hydrogen permeation rates.
2. Experimental
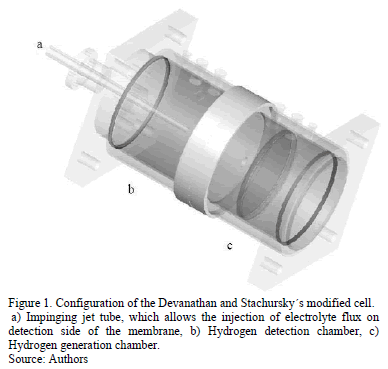
The permeation of hydrogen was made in a modified Devanathan-Stachurski's electrochemical cell developed in our laboratory. This cell allows the injection of an electrolyte jet directly on hydrogen detection side of the permeation membrane. The configuration of the modified cell is showed in Fig. 1.
The cell was maintained at a temperature of 25°C during all measurements. All experiments were conducted using both, with the injection of electrolyte flux and, without application of any flux over detection side of the membrane. Platinum wire and saturated mercurous sulphate (SSE) were used as counter and reference electrodes, respectively. All potentials are hereafter referred to as SSE.
The material studied was iron (99,95%) plates of 20mm x 20mm x 0,25mm. In order to homogenize the iron microstructure, the samples were annealed in vacuum during 1 h at 900 ºC.
The both sides of the iron samples were polished with SiC emery papers until 1500 grain size. The samples were cleaned using ultrasonic cleaning during 5 minutes with toluene, distilled water and finally in alcohol.
Hydrogen was cathodically produced in the input cell and was anodically measured in the output cell with two potentiostats. Cathodic polarization of the entrance side was achieved in a galvanostatic way. The electrolytic solution used in the entrance chamber was NaOH 0,1M + 2mgL-1 As2O3, the cathodic current density used was 7 mAcm-2. A borate buffer solution (pH 8.4) was used in the hydrogen detection side, this solution was chosen based on its capacity to form and reduce an oxide film with some reversibility [19]. The detection side was maintained under potenciostatic control. At the initial time a reduction constant potential (-900 mV) was applied at the exit side during 300 s in order to remove the spontaneously formed oxide on air. After that, a oxidant constant potential (-160 mV) was applied, not only before the beginning of the permeation (in order to allow the passivation current to decrease to a quasi-stationary value) but also during the hydrogen permeation (in order to oxide the emergent hydrogen). The area under evaluation was settled at 0.414 cm2.
3. Results and Discussion
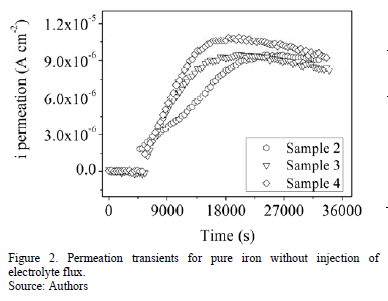
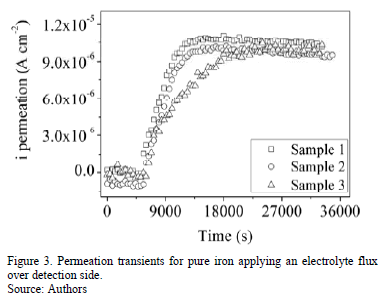
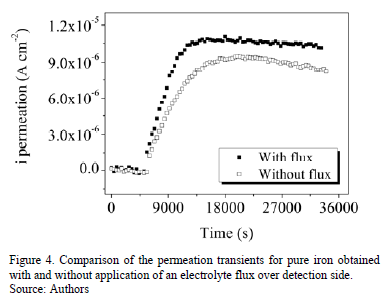
Figs. 2 and 3 shows the hydrogen permeation transients of the samples tested without and with injection of electrolyte flux, respectively. Slightly modifications on hydrogen permeation transients were observed when an electrolyte flow was injected over detection side of pure iron membrane. For instance, higher slope of the current transient and higher steady state current permeation is observed in the experiments performed with electrolyte flux, as can be observed in Fig. 4.
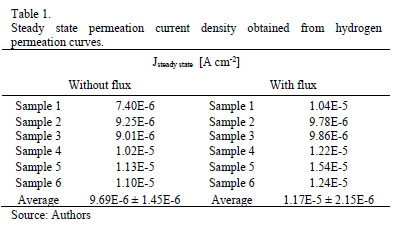
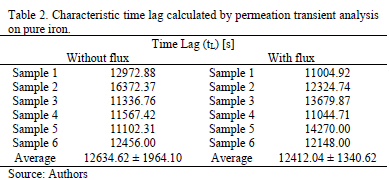
Table 1 and Table 2 present the steady state of permeation current density and the characteristic time lag obtained from hydrogen permeation curves performed in both kinds of experiments. Both, the hydrogen permeation current and the time elapsed to achieve the steady state are in same order of magnitude in the experiments conducted with and without electrolyte flux; however, the average value of the steady state current density is slightly higher and the time lag value is slightly lower when the electrolyte flux was applied over the detection surface.
Taking into account that the passive layer is responsible for the decrease of steady permeation state [14, 20, 25] and that part of the hydrogen which arrives at the detection side can react chemically with the iron oxide [22, 28] reducing the hydrogen flux measured, it is reasonable suppose, that the flux of electrolyte applied over the exit side of the membrane could modify the oxide behavior and consequently the boundary conditions at the exit side.
The time lag (tL) is the time required to obtain a steady-state of the hydrogen flow through the sample after the sudden change of the boundary conditions [3]. Equation (1) shows the relationship between the time lag (tL) and the diffusion coefficient (D), where S is the thickness of the sample [3, 5].
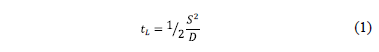
Slightly superior values of tL have been observed in the experiments conducted without application of an electrolyte flux over the exit side of the membranes. The most likely explanation of this result could be related with the barrier effect given by the passive layer of iron oxide formatted on the detection side of the metallic membrane.
Previous research has found that the passive iron oxide film could interact with the emerging hydrogen [22]. The emerging hydrogen finds adequate conditions to auto-oxidize and reduce iron oxide at the surface. XANES measurements showed that the oxide film may change due to their interaction with the emerging hydrogen. This means that the oxide film formed is not completely inert and could be changed due to oxidation / reduction reactions with emerging hydrogen from the metal. To verify the local electrochemical activity between the passive oxide film and the emerging hydrogen in this research electrochemical impedance spectroscopy (EIS) measurements are performed before, during and after the hydrogen permeation in presence of the electrolyte flow on the detection surface.
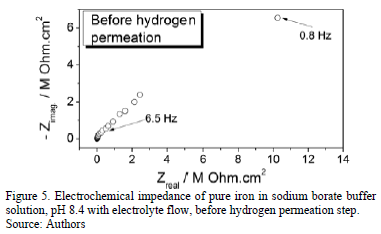
The Electrochemical Impedance Spectroscopy (EIS) is a non-destructive technique usually used to characterize the behavior an electrochemical interface. Therefore it has important applications in evaluating the performance of bare or coated metals in different environments [29]. EIS diagram of pure iron in solution using borate buffer electrolyte flow before the step of hydrogen permeation in the open circuit potential (EOCP) is presented in Figure 5. It is observed that the impedance diagram is constituted by an open capacitive loop, with real and imaginary impedances rather large in the order of MOhms.cm2. This indicates a high stability of the passive oxide layer formed on the metal previous to hydrogen permeation and shows little reactivity of the oxide layer before the hydrogen emerges from the metal.
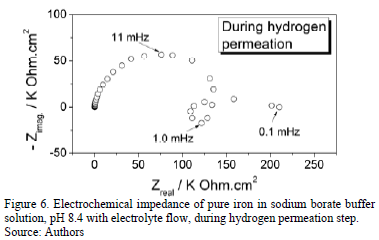
EIS diagram of the metal made during hydrogen permeation at an anodic polarization potential (E = -0.160 V) is presented in Fig. 6. Unlike the EIS pattern obtained before hydrogen permeation, this diagram includes a capacitive loop at a characteristic frequency of 11 mHz, an inductive loop at a characteristic frequency of 1.0 mHz and a capacitive extremely low frequency loop at 0.1 mHz. The impedance of the system at this condition drops by about 100 times compared to the system before permeation. The drop of the resistance in the system is related to an increased reactivity of the passive oxide layer to interact with the emerging hydrogen. In fact new electrochemical extremely slow processes are evidenced by the impedance, according to the low frequency inductive loop (1.0 mHz) and a capacitive loop to extreme low frequency (0.1 mHz) observed in EIS diagram of the Fig. 6. The inductive loop may be related to dissolution of iron or reduction of the passive oxide layer. Reducing Fe3+ to Fe2+ in the passive iron oxide layer has been demonstrated using in situ XANES measurements [22]. The extreme low frequency capacitive loop is related to the adsorption/desorption of emerging hydrogen and its subsequent oxidation H0/H+ at the surface during detection process.
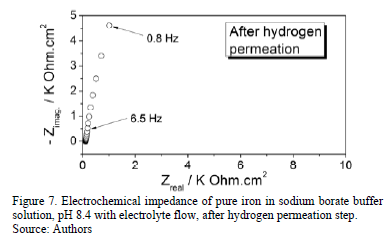
Electrochemical impedance measurement performed after the hydrogen permeation is presented in Fig. 7. It is observed that the impedance returns to its original state, showing a single open capacitive loop. This indicates that the reactivity of the surface becomes low again when the hydrogen permeation process is suspended and there is not emerging hydrogen from the metal surface.
The passive film formed in the iron surface in contact with the electrolyte acts like as barrier to the hydrogen [4, 5], this barrier layer has low permeability and retards the diffusion of hydrogen trough the sample thickness and consequently, the time necessary to achieve the stationary concentration may increase substantially [1-6]. The observed differences, both of the steady state current and the tL values, indicates that the passive film could be modified during the permeation test when a flux of electrolyte was injected over the detection side. It is probably that the thickness of the passive layer is decreased when an electrolyte flow is applied. Consequently, higher steady state current density and lower time lag values could be observed. The consequences of these phenomena could be tremendous when mass transport and material properties wish to be calculated from permeation curves. This has not been taken in account in many papers published in this topic [4-6].
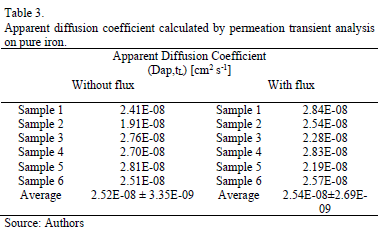
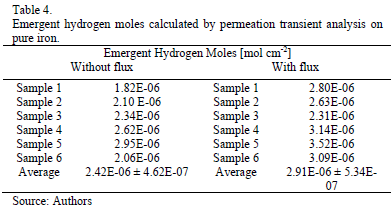
The permeation curves analysis allows calculating the hydrogen permeation parameters: apparent diffusion coefficient and the emerging quantity of hydrogen at the exit side. A concentration change in the metal near the surface is monitored by changes in the electrochemical current of the surface, and a constant penetration of hydrogen, j (mole H cm-2 s-l), into the metal due to the electrochemical deposition can be measured according to equation (2):
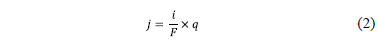
Where i is the current (A), F the Faraday constant and q the sample surface area. The time integral of the current yields the total amount of hydrogen absorbed by the metal. The parameters calculated are compiled in the tables 3 and 4.
The apparent diffusion coefficient, when the value was calculated by the time lag method (equation1) [3], is slightly higher in the experiments carried out applying an electrolyte flux, this result is in concordance with the raise observed in the steady-state permeation current in this experimental condition. Similarly, the quantity of hydrogen that diffuses and reaches the exit side calculated from the area under the permeation curve is slightly higher when an electrolyte flux was injected.
The differences found in the permeation parameters are in concordance with the behavior observed on the permeation curves. The results obtained show that the iron oxide formed on the metal surface could be modified by applying an electrolyte flux during hydrogen permeation tests. However, the differences found in the permeation parameters are not significant in order to establish the role of the iron oxide passive layer on the hydrogen permeation rates. According to that, the results are not conclusive and other electrochemical techniques may be used in order to evaluate and clear up the changes that occurred at the iron surface and the role of the passive layer during hydrogen permeation.
4. Conclusions
Some differences were observed at the hydrogen permeation transients because applications of an electrolyte flux over the detection side of the pure iron membranes. A slightly increase of the steady state permeation current is obtained when an electrolyte is injected over the exit side. A delay of the time lag, which characterizes hydrogen permeation, was observed without electrolyte flux injection; the value of the time lag, tL is slightly higher in the experiments conducted without electrolyte flux. The calculated permeation parameters (Dap, tL and emergent hydrogen moles) are slightly higher in the tests in which an electrolyte flux was applied. The results obtained show that the oxide surface of iron could be modified by the flux of electrolyte applied during hydrogen permeation tests. However, these preliminary results are not conclusive and did not allow understanding the mechanisms involved in this changes.
Acknowledgements
Authors are pleased to acknowledge the financial assistance of the CODI - Universidad de Antioquia through the project N° MDC10-1-03 and "Estrategia de Sostenibilidad 2013-2014. One of the authors (Pérez-Ceballos) is grateful with the Enlanza Mundos program of the Medellin Municipality and LNDC laboratory by the financial support during her stay in Rio de Janeiro-Brasil.
References
[1] Addach, H. Bercot, P. Rezrazi, M. and Takadoum, J., Study of the electrochemical permeation of hydrogen in iron, Corrosion Science, 51, pp. 263-267, 2009. [ Links ]
[2] Addach, H. Bercot, P. Rezrazi, M. and Wery, M., Hydrogen permeation in iron at different temperatures, Materials Letters, 59, pp. 1347-1351, 2005. [ Links ]
[3] Boes, N. and Züchner, H., Electrochemical methods for studying diffusion, permeation and solubility of hydrogen in metals, Journal of Less Common Metals, 49, pp. 223-240, 1976. [ Links ]
[4] Brass, A.M. and Collet-Lacoste, J.R., On the mechanism of hydrogen permeation in iron in alkaline medium, Acta Materialia, 46, pp. 869-879, 1998. [ Links ]
[5] Pyun, S.-I. and Oriani, R.A., The permeation of hydrogen through the passivating films on iron and nickel, Corrosion Science, 29, pp. 485-496, 1989. [ Links ]
[6] Schomberg, K., Riecke, E. and Grabke, H.J., Hydrogen permeation through passive films on iron, Paris, France, 1993. [ Links ]
[7] Zakroczymski, T., Electrochemical determination of hydrogen in metals, Journal of Electroanalytical Chemistry, 475, pp. 82-88, 1999. [ Links ]
[8] Zakroczymski, T., Adaptation of the electrochemical permeation technique for studying entry, transport and trapping of hydrogen in metals, Electrochimica Acta, 51, pp. 2261-2266, 2006. [ Links ]
[9] Devanathan, M.A. and Stachurski, Z., The adsorption and diffusion of electrolytic hydrogen in palladium, Proc. R. Soc. London Ser. A 270 1962, P.90. [ Links ]
[10] Wang, M.Q., Akiyama, E. and Tsuzaki, K., Hydrogen degradation of a boron-bearing steel with 1050 and 1300MPa strength levels, Scripta Materialia, 52 (5), pp. 403-408, 2005. [ Links ]
[11] Hardie, D. and Liu, S., The effect of stress concentration on hydrogen embrittlement of a low alloy steel, Corrosion Science, 38 (5), pp. 673-802, 1996. [ Links ]
[12] Heubaum, F.H. and Berkowitz, B.J., The effect of surface oxides On the hydrogen permeation through steels, Scripta Metallurgica, 16, pp. 659-662, 1982. [ Links ]
[13] Lee, J.L., Waber, J.T. and Park,Y.K., The effect of mechanical polishing on hydrogen permeation in iron single crystal, Scripta Metallurgica, 20, pp. 823-828, 1986. [ Links ]
[14] Manolatos, P., Jerome, M., Duret-Thual, C. and Le Coze, J., The electrochemical permeation of hydrogen in steels without palladium coating, Part 1: Interpretation difficulties, Corrosion Science, 37 (1), p. 1773-1783, 1995. [ Links ]
[15] Oriani, R.A., The diffusion and trapping of hydrogen in steel, Acta Metallurgica, 18, pp. 147-157, 1970. [ Links ]
[16] Leblond, J.B., and Dubois, D., A general mathematical description of hydrogen diffusion in steels Part I. Derivation of diffusion equations from boltzmann-type transport equations, Acta Metallurgica, 31, pp. 1459-1469, 1983. [ Links ]
[17] Leblond, J.B. and Dubois, D., A general mathematical description of hydrogen diffusion in steels, Part II. Numerical study of permeation and determination of trapping parameters, Acta Metallurgica, 31, pp. 1471-1478, 1983. [ Links ]
[18] Wu, E., A mathematical treatment of the electrochemical method of hydrogen permeation and its application in hydrogen traps and embrittlement, Journal of the Electrochemical Society, 134 (9), pp. 2126-2132, 1987. [ Links ]
[19] Zhang,T.Y., Zheng, Y.P. and Wu, Q.Y., On the boundary conditions of electrochemical hydrogen permeation through iron, Journal of the Electrochemical Society, 146, pp. 1741-1750, 1999. [ Links ]
[20] Manolatos,P., Le Coze, J., and Duret-Thual, C., Electrochemical permeation of hydrogen in iron - Influence of the passive layer formed on the detection side, Comptes Rendus De L Academie Des Sciences Serie Ii, 306, 1988. [ Links ]
[21] Manolatos, P., Jerome, M. and Galland, J., Necessity of a palladium coating to ensure hydrogen oxidation during electrochemical permeation measurements on iron, Electrochimica Acta, 40 (7), pp. 803-937, 1995. [ Links ]
[22] Modiano, S., Carreno, J., Fugivara, C.S., Torresi, R.M., Vivier, V., Benedetti, A., et al., Changes on iron electrode surface during hydrogen permeation in borate buffer solution, Electrochimica Acta, 53, pp. 3670-3679, 2008. [ Links ]
[23] Modiano, S., Carreño, J.A., Fugivara, C.S., Benedetti, A.V. and Mattos, O.R., Effect of hydrogen charging on the stability of SAE 10B22 steel surface in alkaline solutions, Electrochimica Acta, 51, pp. 641-648, 2005. [ Links ]
[24] Bruzzoni, P. and Garavaglia, R., Anodic iron-oxide films and their effect on the hydrogen permeation through steel, Corrosion Science, 33, pp. 1797-1807, 1992. [ Links ]
[25] Bruzzoni, P. and Riecke, E. On the mechanism of hydrogen transport through the passive oxide film on iron, Corrosion Science, 36, pp. 1597-1614, 1994. [ Links ]
[26] Bruzzoni, P., Efectos de superficie en la difusión de hidrógeno en hierro y aleaciones ferrosas, Dr. Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2003. [ Links ]
[27] Ash, R., Barrer, R.M., and Palmer, D.G., Diffusion in multiple laminates, British Journal of Applied Physics, 16 (6), pp. 873-875, 1965. [ Links ]
[28] Oblonsky, L.J., Ryan, M.P. and Isaacs, H.S., In situ XANES study of the formation and reduction of the passive film formed on Fe in acetate solution, Corrosion Science, 42, pp. 229-241, 2000. [ Links ]
[29] Piratoba-Morales, U., Vera-López, E. y Ortiz-Otálora, C., Aspectos básicos en la interpretación de diagramas de impedancia electroquímica, DYNA, 77 (162), pp. 13-19, 2010. [ Links ]
A.M. Pérez-Ceballos, received the Bs. Eng in Metallurgical Engineering in 2001 from the Universidad de Antioquia. Medellín, Colombia, the MSc. degree in Science and Technology of Materials in 2006 from Instituto Sabato, Universidad de General San Martin, Comisión Nacional de Energia Atómica, Buenos Aires, Argentina; nowadays is DSc. candidate in Engineering from the Universidad de Antioquia, (Medellin, Colombia. From 2006 to 2012, she worked as Full Professor in the Materials Engineering Department, Facultad de Ingeniería, Universidad de Antioquia, Colombia. Currently, she is Metallurgical Engineering for Friatec do Brasil, manufacturing industry of high corrosion resistant pumps.
J.A. Calderón-Gutiérrez, received the Bs. Eng. in Metallurgical Engineering in 1992, the MSc. degree in Chemical Science in 1998, both of them from Universidad de Antioquia, Medellín, Colombia, the Dr. degree in Metallurgical and Materials Engineering, in 2003, from Universidade Federal de Rio do Janeiro, Brazil. He works as Full Professor in the Materials Engineering Department, Facultad de Ingeniería, Universidad de Antioquia, Colombia.
O. Rosa-Mattos, received the Bs. Eng. in Metallurgical Engineering in 1975, the MSc. degree in Chemical Science in 1977, both from Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, the PhD. in Physique Des Liquides in 1981, from Universite de Paris VI (Pierre et Marie Curie), Paris, France. He works as Full Professor in the Metallurgical and Materials Engineering Department, COPPE, Universidade Federal do Rio de Janeiro, Brasil.