Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.82 no.189 Medellín Jan./Feb. 2015
https://doi.org/10.15446/dyna.v82n189.42954
http://dx.doi.org/10.15446/dyna.v82n189.42954
Structural modification of regenerated fuller earth and its application in the adsorption of anionic and cationic dyes
Modificación estructural de la tierra fuller regenerada y su aplicación en la adsorción de colorantes catiónicos y aniónicos
Oscar Dario Beltran-Pérez a,Angelina Hormaza-Anaguano b,Benjamin Zuluaga-Diaz c & Santiago Alonso Cardona-Gallo d
a Facultad de Minas, Universidad Nacional de Colombia, Medellín, Colombia, odbeltranp@unal.edu.co
b Facultad de Ciencias, Universidad Nacional de Colombia, Medellín, Colombia, ahormaza@unal.edu.co
c Facultad de Minas, Universidad Nacional de Colombia, Medellín, Colombia, bzuluagad@unal.edu.co
d Facultad de Minas, Universidad Nacional de Colombia, Medellín, Colombia, scardona@unal.edu.co
Received: April 7th, 2014. Received in revised form: September 11th, 2013. Accepted: September 25th, 2014.
Abstract:
Fuller's earth is an inorganic material used in the electric industry for dielectric oil regeneration. After decontamination treatment, Fuller's earth is regenerated and reused. In this study, the removal capacity of regenerated Fuller for the treatment of water contaminated with dyes of different nature and in turn, how this capacity can be increased by structural modification of the material was evaluated. Thus, the regenerated Fuller earth without any modification reached 99% removal of methylene blue. However, for treatment of effluents contaminated with red 40, acid and thermal modification of material was required to obtain adsorption greater than 94% of the dye. In conclusion, regenerated Fuller earth adsorbent is suitable for treating effluents contaminated with cationic coloring materials. However, removal of anionic dyes is not efficient, the structural modification of the material to be necessary to enhance the removal of these type colorants.
Keywords: Regenerated Fuller earth, methylene blue, red 40, acid modification, thermal modification, cationic and anionic dyes.
Resumen:
La tierra Fuller es un material inorgánico usado en la industria eléctrica para la regeneración del aceite dieléctrico. Después de un tratamiento de descontaminación, la tierra Fuller se regenera y reutiliza. En este estudio se evaluó la capacidad de remoción de la tierra Fuller regenerada para el tratamiento de aguas contaminadas con colorantes de diferente naturaleza y a su vez, como esta capacidad puede ser aumentada mediante la modificación estructural del material. Así, la tierra Fuller regenerada sin ninguna modificación alcanzó remociones del 99% de azul de metileno. Sin embargo, para el tratamiento de efluentes contaminados con rojo 40 se requirió la modificación acida y térmica del material para obtener adsorciones superiores al 94% del colorante. En conclusión, la tierra Fuller regenerada es un material adsorbente adecuado para el tratamiento de efluentes contaminados con colorantes de naturaleza catiónica. No obstante, la remoción de colorantes aniónicos no es eficiente, siendo necesaria la modificación estructural del material para mejorar la remoción de este tipo de colorantes.
Palabras Clave: Tierra Fuller regenerada, azul de metileno, rojo 40, modificación ácida, modificación térmica, colorantes catiónicos y aniónicos.
1. Introduction
Current population growth with the consequent increase in demand for water resources, extreme environmental conditions caused by climate change around the world and depletion and contamination of water sources available for human consumption have increased interest and concern both the authorities and the population for the quality and proper management of water resources. More stringent regulations and controls have been developed, but their implementation is slow and the problems associated with the depletion and pollution of water resources continue [1]. Wastewater from industry has become the main cause of pollution of water sources due to its high volumes and variety of compounds present in them [2,3]. The presence of recalcitrant compounds and difficult to degrade as the dyes in water sources is a latent problem that tends to increase due to the wide variety and uses of these compounds in the industry, generating increasing impacts on the environment and ecosystems [4].
The dye in the water affects the resource and its various ecosystems; the consequences are the reduction in the penetration of solar radiation and hence the decrease of the photosynthetic process that contributes to the preservation and quality of the resource [5,6]. On the other hand, the reduction of dissolved oxygen in the water and accumulation in the food chain of some of these compounds and their degradation products can have serious effects on humans, animals and aquatic species [7-9]. The physical appearance and characteristic of the water is also affected; small dye concentrations can generate visible effects in making resource unfit for human consumption [10]. In this regard, various technologies aimed at treating industrial effluents have been developed more quickly [11], especially those related to the removal of recalcitrant compounds as dyes [4]. Some conventional methods such as coagulation, oxidation, photocatalytic and degradation by microorganisms have been developed for the treatment of dyes; however, these approaches have shown little efficiency [10,12], because, the non-biodegradable nature, strength and high stability of these compounds [13-16]. Adsorption processes have efficient removals and advantages in implementation, operation and costs, making this physicochemical technique most commonly used for the treatment of water contaminated with dyes [10,12,17]. For a more economically favorable process, a large number of alternative materials as the activated carbon have been used in adsorption [18-20]. In contrast to activated carbon, clays have a lower cost [21]. Different types of clays, including Fuller's earth have been used in the removal of dyes [16,22,23]. The structure of these materials has been amended in several investigations in order to improve their adsorptive properties [24-26].
Fuller's earth is an aluminum silicate composed mainly by dioctahedral smectites (montmorillonite), natural zeolites (analcime) and sepiolite (loughlinite) [22,27]. In general, the structure of the material has a three-dimensional array, consisting of successive planes of oxygen (O) and hydroxyl (OH) connected or linked to elements such as silicon (Si), aluminum (Al), magnesium (Mg) and other cations, forming layers of tetrahedral silicon and octahedral aluminum or magnesium bound oxygens and hydroxyls [28,29]. Fuller's earth has been used in bleaching and refining of oils, fats and waxes for a long time [30,31]. Currently, it is used in the electric industry for the regeneration of the dielectric oil in power transformers. Once regenerated the dielectric oil, Fuller earth is impregnated with part of the oil and by-products generated by the oxidation of it, making the Fuller earth a hazardous waste [32]. Earlier, this contaminated material was deposited in a conventional landfill, ignoring the consequences that this type of hazardous waste can cause in the environment. Alternatively, the residue was subjected to an incineration process, despite the potential risk of transformation into dioxins and furans (even more toxic than the original waste compounds) of some of the compounds containing the contaminated material [32,33]. From the technique of solid-liquid extraction using as solvent ethanol is achieved decontaminate and regenerate Fuller's earth to get back their properties as adsorbent material and extend its life cycle, making it possible to reuse in this case for the treatment of water contaminated with dyes [34,35].
Thus, the purpose of this study is to explore the possibility of using regenerated Fuller earth as adsorbent for the removal of cationic and anionic dyes. For this, the adsorptive capacity of the regenerated Fuller earth for the treatment of water contaminated with the dyes methylene blue and red 40 was evaluated, and in turn, is set as this capacity can be increased from the acid and thermal modification of the material. Therefore, a material of great untapped potential adsorbent is reused and partly contributes to solve the serious problems caused by the presence of dyes in water sources.
2. Materials and methods
2.1. Structural analysis
In order to check the structural and morphological changes produced by each of the modifications made on native and regenerated Fuller earth, was determined in the modified samples their composition, morphology, point of zero charge and adsorptive capacity.
The results of this analysis were compared with the native and regenerated Fuller earth unmodified. To determine the point of zero charge, the pH value and the adjustment thereof is carried out with the automatic titrator Titrino Plus 848 of Metrohm a 24°C. The chemical and morphological analysis of the material were performed at the Laboratory for Advanced Microscopy, Facultad de Minas, using energy dispersive detector coupled to a scanning electron microscope, equipment SEM JEOL JSM 5910 LV with an accelerating voltage of 15 kV, prior metallization of the samples with gold. With respect to the adsorption capacity, the amount of dye removed by the native material, regenerated and modified determined using a spectrophotometer UV/Vis Lambda 35 at wavelength of maximum absorption of lmáx = 665 nm for methylene blue and lmáx = 502 nm for red 40, used glass cell of 1 cm length.
2.2. Adsorbent material preparation
Fuller's earth used in this research was provided by the local distributor of the material in Medellin, Colombia. Fuller's earth contaminated with dielectric oil was decontaminated according to [34], through the technique of solid-liquid extraction using ethanol as solvent. The material thus decontaminated and recovered was used in the various tests carried out in this investigation.
2.2.1. Acid and thermal modification
In general, the chemical and physical properties of clays can be modified to increase their natural capacity adsorbent and provide specific characteristics for certain applications. The modification can be achieved by various treatments, generating an effect on physicochemical and mineralogical properties of the material [36]. Acid and thermal modifications are the techniques most commonly employed for such transformation. Changes in the mineralogical structure and chemical composition of the material depend on its nature and the conditions of treatment, as residence time, temperature and concentration of the medium [37]. The acid modification of the native and regenerated Fuller's earth was performed with 6.0 M HCl as described by [25]. 5.0 g of the material was mixed with 500 mL of a 6.0 M solution of HCl in a system under reflux at temperature of 368 K for 24 hours with occasional stirring of the mixture. After the modification, the resulting mixture was filtered and dried in an oven at 313 K. The thermal modification of the material is worked at temperature of 378 K.
2.2.2. Dilute acid modification
The pH is a critical variable in the removal processes as influences the adsorption at the solid-liquid interface of the different species presents in the medium [38]. The variation of the pH value of the solution can significantly affect the surface charge of the material and the degree of ionization and speciation of the adsorbate [23]. Native and regenerated Fuller earth was modified. 0.5 g of the material was mixed with 50 mL of water previously adjusted with 0.1 M HCl to a pH value equal to 2.0. The resulting mixture remained in contact for a period of three hours under continuous stirring and room temperature in triplicate.
2.3. Determination of point of zero charge
The surface of any material is sensitive to changes in pH of the medium. This generates surfaces charged positively or negatively. In general, if the pH of the medium is greater than the point of zero charge of the solid (pH value at which the surface of the material has a net charge of zero), it has a predominance of negative charges in the material. Conversely, if the pH of the medium is lower than the point of zero charge, positively charged solid particles is obtained [39,40]. Thus, the point of zero charge can establish the affinity of the material for the various ionic species present in the medium. Determination of point of zero charge (PZC) was the methodology used to establish the polarity of the surface of native and regenerated material. And so verify the change on their structure conducted after the respective modifications. This PZC was obtained from the method of pH drift. 0.5 g of the material was mixed with 50 mL of water adjusted with 0.1 M HCl and 0.1 M NaOH at different pH values (2, 3, 4, 5 and 6). Subsequently, the initial and final pH of each sample was measured, after 48 hours under continuous stirring and room temperature. This procedure was performed in triplicate for each sample.
2.4. Determination of adsorption capacity
Acid and thermal treatment can alter the adsorptive capacity of the material [41]. The adsorbent capacity of the native, regenerated and modified Fuller earth was evaluated in a batch system, for 24 hours at room temperature and a solution-material relationship of 100 mLg-1, that is, 0.5 g of adsorbent material was mixed with 50 mL of a simulated effluent of methylene blue at 80 ppm. The adsorption capacity of the materials was also evaluated with a simulated effluent of red 40 at 30 ppm under the same conditions referred before. Subsequently, the samples were centrifuged at 3000 rpm for 5 minutes. The dye concentration in each sample was determined, after checking that there are not changes in the absorption spectrum of the respective dyes, from the calibration curves previously drawn on the spectrophotometer.
3. Results and discussion
3.1. Morphological analysis
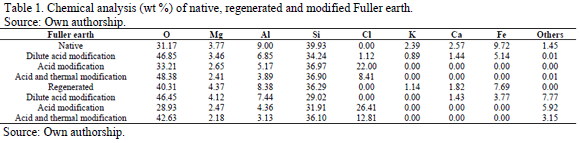
The acid treatment is one of the most common chemical modifications. In general, the acid activation process consists of two stages: first, the substitution of the interlayer cations for protons and posteriorly, the partial dissolution of Al, Mg and Fe from the octahedral sheet and to a lesser extent, Si from the tetrahedral sheet as a result of dehydroxylation of structural OH groups of the material [11,29,42]. The gradual dissolution of these cations leads to its decreasing in the material, which is checked by chemical analysis (Table 1). The relative increase observed in the silicon content of the modified materials depends on the dissolution of structural and exchangeable cations [29], being these cations more susceptible to acid attack than the SiO4 and SiO3OH groups from the tetrahedral sheet of the material [26]. Acid modification is partly responsible for the morphological change of the material. The micrographs taken to the materials before and after conducted the respective modifications are presented in Fig. 1.
The micrographs show the surface of the regenerated Fuller earth more stressed than the surface of native Fuller earth, due to the decontamination treatment which this material was initially submitted. By comparing the morphology of native, regenerated and modified Fuller earth, is seen as the modified Fuller earth has a more altered, worn and peeling surface than the other samples, being this effect larger in the acid and thermal modified Fuller earth than the only acid modified Fuller earth. This is due to cracks and cavities between the sheets of the material generate by the thermal treatment, as a result of the release of water absorbed in the pores and on the surface of the material, besides of the elimination of carbonates and other oxides that can be found as impurities [43].
3.2. Effect on the point of zero charge
In general, a reduction is observed in the value of the point of zero charge of the native and regenerated Fuller earth with different modifications. This result is presented in the Table 2.
The less point of zero charge of the regenerated Fuller earth regarding native Fuller earth is partly due to the decontamination treatment to which the material is initially subjected. In the case of native Fuller earth, its point of zero charge changes from an initial value of 7.1 to 3.3 with the acid modification and then to a value of 2.1 with the thermal treatment (Table 2). This latter result is consistent with that reported by [25] who found a point of zero charge of 1.8 for a sample of Fuller earth similarly modified. The behavior of regenerated Fuller earth is similar to the native Fuller earth.
This material reduced its initial point of zero charge from 6.6 to 2.3 after the respective acid and thermal modification. Thus, at pH values below the point of zero charge, the particles on the surface of the adsorbent will be positively charged; at pH values above will be negatively charged. Thus, the lowest point of zero charge of the modified materials with respect to the original material evidences the changes generated on its surface and allows in theory, establishes that native and regenerated Fuller earth modified by acid and thermal treatment exhibits greater affinity, due to their surface charge, by anionic dyes such as red 40.
Modification of the materials with water adjusted to a pH of 2.0 was sufficient to reduce the point of zero charge of native and regenerated Fuller earth to a value equal to 4.0. This result shows the sensitivity of the material with pH adjustment using HCl 0.1 M and a short contact period.
3.3. Effect on the adsorbent capacity
The adsorption of methylene blue and red 40 dyes on native, regenerated and modified Fuller earth is explained based on the two types of interactions that occur between the dye molecules and the surface of the material: the isomorphic substitution and protonation-deprotonation reactions.
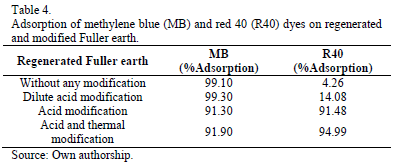
The internal and external surface of the Fuller earth and its permanent negative charge provide it with a special affinity for cationic dyes as methylene blue [22,25]. The permanent negative charge of the material is derived from isomorphic substitutions that occur in its structure regardless of environmental conditions [44]. The substitution generates an imbalance of charge due to the replace of Al+3 by Mg+2 and Fe+2 in the octahedral sites and Si+4 by Al+3 in the tetrahedral sites, and causes each tetrahedron of [AlO4]-5 has a net negative charge [45]. The total neutrality of the structure is preserved balancing each tetrahedron [AlO4]-5 with a positive charge provided by interchangeable cations (K+, Na+, Ca+2, Mg+2) electrostatically attached to the material structure. These cations have great freedom of movement and can even be exchanged for other cations. Excess of negative charges make the extremely acid surface thus favoring the adsorption of positively charged particles such as methylene blue cationic dye. This explains why the adsorption of methylene blue on native and regenerated Fuller earth naturally occurs with high percentages of removal, the structural modification of the material is unnecessary to achieve 98% higher adsorptions on both materials.
Tables 3 and 4 shows that the modification of the material slightly decreases the percentage of adsorption of methylene blue dye because the lowest point of zero charge of the modified materials favors on the surface a positive charge repulsion generated by the similarly charged species. So, lower adsorption achieved with the modified materials is due in part to the strong repulsion that occurs between the dye molecules and the positively charged sites on the surface, and the competition between H+ ions and the dye molecules by available negatively charged sites on the material [25,38].
Another type of interaction between Fuller earth and dye molecules is related to the Si-OH and Al-OH or Mg-OH groups found on Fuller's earth surface. These OH groups can have a positive or negative charge depending on the type of metal ions at which are united, and the pH of the solution [40]. Depending on the pH value of the medium, the hydroxyl groups can be protonated or deprotonated [46]. At low pH values, sites acquire a positive charge due to the presence of protonated groups AlOH2+ and SiOH2+, as a result, a strong interaction and adsorption of negative charges are generated. Conversely, with an increase in the pH value, these sites reduce their charge to a neutral value and then at higher pH values develop a negative charge according to equations 1 and 2.
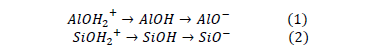
Thus, and as previously noted with the points of zero charge of native and regenerated Fuller earth modified, the modification generates a positive charge on the structure of the material that leads to an increase in the adsorption percentage of red 40 dye. Furthermore, in these materials, the negative charge of silica sites is balanced by ions of H+ produced during modification. This reduces the diffusion difficulties of molecules of the dye and generates a significant increase in electrostatic attractions between the negative charges of the anionic dye and the positive charged sites of alumina and manganese dioxide, with a consequent increase in the removal of dye. As the pH of the medium increases, the number of positively charged sites is reduced and therefore, the adsorption of the dye.
It was found that native and regenerated Fuller earth without any modification has an adsorption percentage of 40 red almost nil. However, once modified, color removal was higher than 94% for both materials. The lower adsorption capacity of the red 40 on the native and regenerated Fuller earth without any modification is due to the ionic repulsion of the anionic dye molecules and the surface of the material. Furthermore, the presence of OH-ions in solution creates a competitive environment with the dye ions to positively charged sites on the material that decreases the adsorption percentage. The results show the affinity of the native and regenerated Fuller earth to cationic dyes and make clear the need to modify these materials for efficient removal of anionic dyes.
4. Conclusions
Fuller's earth retains and enhances its properties as adsorbent material after decontamination treatment. Its potential for reuse, in this particular case for the treatment of water contaminated with dyes was proved. Due to the characteristics of the material and its point of zero charge, native and regenerated Fuller earth are suitable materials for removing cationic dyes such as methylene blue due to its surface having affinity for dyes of this type. For removal of anionic dyes, the structural modification of the material is required [47].
Acknowledgements
The authors express gratitude to Universidad Nacional de Colombia Sede Medellín for the infrastructure support of the Laboratory of Experimental Chemistry and Hydraulics Laboratory. Likewise, Professor Medardo Pérez by Chemical Analysis and Micrographs taken to the materials and Dirección de Investigación Sede Medellín, DIME, for grant the research project QUIPU 201010011038.
References
[1] Daifullah, A. and Girgis, B., Impact of surface characteristics of activated carbon on adsorption of BTEX, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 214 (1-3), pp. 181-193, 2003. http://dx.doi.org/10.1016/S0927-7757(02)00392-8 [ Links ]
[2] Gupta, V.K and Suhas, Application of low-cost adsorbents for dye removal - A review, Journal of Environmental Management, 90 (8), p. 2313-2342, 2009. http://dx.doi.org/10.1016/j.jenvman.2008.11.017 [ Links ]
[3] Alemán-Romero, A.L., Evaluación de la esterificación sobre cascarilla de arroz como estrategia para incrementar la capacidad de remoción del colorante rojo básico 46, Medellín, 2012. [ Links ]
[4] Che-Galicia, G., Remoción de un colorante de los efluentes de la industria textil mediante adsorción en una zeolita natural, México D.F., 2011. [ Links ]
[5] McKay, G., Otterburn, M.S. and Sweeney, A.G., The removal of colour from effluent using various adsorbents - III. Silica: Rate process, Water Research, 14 (1), pp. 15-20, 1980. http://dx.doi.org/10.1016/0043-1354(80)90037-8 http://dx.doi.org/10.1016/0043-1354(80)90038-X [ Links ]
[6] Davis, J., American Dyestuff Reporter, 80 (3), 19 P, 1991. [ Links ]
[7] Slokar, Y.M. and Le Marechal, A.M., Methods of decoloration of textile wastewaters, Dyes and pigments, 37 (4), pp. 335-356, 1998. http://dx.doi.org/10.1016/S0143-7208(97)00075-2 [ Links ]
[8] Zollinger, H., Color chemistry: Syntheses, properties and applications of organic dyes and pigments, Wiley-VCH, 2003. [ Links ]
[9] Wang, S., Boyjoo, Y., Choueib, A. and Zhu, Z., Removal of dyes from aqueous solution using fly ash and red mud, Water Research, 39, pp. 129-138, 2005. http://dx.doi.org/10.1016/j.watres.2004.09.011 [ Links ]
[10] Garg, V., Amita, M., Kumar, R. and Gupta, R., Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: A timber industry waste, Dyes and Pigments, 63, (3), pp. 243-250, 2004. http://dx.doi.org/10.1016/j.dyepig.2004.03.005 [ Links ]
[11] Tuesta, E.G., Vivas, M., Sun R. y Gutarra, A., Modificación química de arcillas y su aplicación en la retención de colorantes, Revista de la Sociedad Química del Perú, 71, (1), pp. 26-36, 2005. [ Links ]
[12] Chowdhury, S. and Saha, P., Sea shell powder as a new adsorbent to remove Basic Green 4 (Malachite Green) from aqueous solutions: Equilibrium, kinetic and thermodynamic studies, Chemical Engineering Journal, 164, (1), pp. 168-177, 2010. http://dx.doi.org/10.1016/j.cej.2010.08.050 [ Links ]
[13] O'Neill, C., Hawkes, F.R., Hawkes, D.L., Lourenco, N.D., Pinheiro, H.M. and Delée, W., Colour in textile effluents - sources, measurement, discharge consents and simulation: a review, Journal of Chemical Technology and Biotechnology, 74, pp. 1009-1018, 1999. http://dx.doi.org/10.1002/(SICI)1097-4660(199911)74:11<1009::AID-JCTB153>3.0.CO;2-N
[14] Namasivayam, C., Yamuna, R.T. and Arasi, D.J.S.E., Removal of procion orange from wastewater by adsorption on waste red mud, Separation Science and Technology, 37 (10), pp. 2421-2431, 2002. http://dx.doi.org/10.1081/SS-120003521 [ Links ]
[15] Waranusantigul, P., Pokethitiyook, P., Kruatrachue, M. and Upatham, E., Kinetics of basic dye (methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza), Environmental Pollution, 125, pp. 385-392, 2003. http://dx.doi.org/10.1016/S0269-7491(03)00107-6 [ Links ]
[16] Eren, E. and Afsin, B., Investigation of a basic dye adsorption from aqueous solution onto raw and pre-treated sepiolite surfaces, Dyes and Pigments. 73, pp. 162-167, 2007. http://dx.doi.org/10.1016/j.dyepig.2005.11.004 [ Links ]
[17] Doğan, M. and Alkan, M., Adsorption kinetics of methyl violet onto perlite, Chemosphere, 50 (4), pp. 517-528, 2003. http://dx.doi.org/10.1016/S0045-6535(02)00629-X [ Links ]
[18] Nassar, M.M. and El-Geundi, M.S., Comparative cost of colour removal from textile effluents using natural adsorbents, Journal of Chemical Technology and Biotechnology, 50, pp. 257-264, 1991. http://dx.doi.org/10.1002/jctb.280500210 [ Links ]
[19] Singh, B.K. and Rawat, N.S., Comparative sorption equilibrium studies of toxic phenols on flyash and impregnated flyash, Journal of Chemical Technology and Biotechnology, 61, pp. 307-317, 1994. http://dx.doi.org/10.1002/jctb.280610109 http://dx.doi.org/10.1002/jctb.280610405 [ Links ]
[20] Özacar, M. and Şengil, I., Adsorption of reactive dyes on calcined alunite from aqueous solutions, Journal of Hazardous Materials, 98 (1-3), pp. 211-224, 2003. http://dx.doi.org/10.1016/S0304-3894(02)00358-8 [ Links ]
[21] Juang, R., Wu, F. and Tseng, R., The ability of activated clay for the adsorption of dyes from aqueous solutions, Environmental Technology, 18, pp. 525-531, 1997. http://dx.doi.org/10.1080/09593331808616568 [ Links ]
[22] Atun, G., Hisarli, G., Sheldrick, W. and Muhler, M., Adsorptive removal of methylene blue from colored effluents on fuller's earth, Journal of Colloid and Interface Science, 261, pp. 32-39, 2003. http://dx.doi.org/10.1016/S0021-9797(03)00059-6 [ Links ]
[23] Özcan, A.S., Erdem, B. and Özcan, A., Adsorption of acid blue 193 from aqueous solutions onto Na-bentonite and DTMA-bentonite, Journal of Colloid and Interface Science, 280, pp. 44-54, 2004. http://dx.doi.org/10.1016/j.jcis.2004.07.035 [ Links ]
[24] Eren, E., Investigation of a basic dye removal from aqueous solution onto chemically modified Unye bentonite, Journal of Hazardous Materials, 166, pp. 88-93, 2009. http://dx.doi.org/10.1016/j.jhazmat.2008.11.011 [ Links ]
[25] Hisarli, G., The effects of acid and alkali modification on the adsorption performance of fuller's earth for basic dye, Journal of Colloid and Interface Science, 281, pp. 18-26, 2005. http://dx.doi.org/10.1016/j.jcis.2004.08.089 [ Links ]
[26] Steudel, A., Batenburg, L., Fischer, H., Weidler, P. and Emmerich, K., Alteration of non-swelling clay minerals and magadiite by acid activation, Applied Clay Science, 44, pp. 95-104, 2009. http://dx.doi.org/10.1016/j.clay.2009.02.001 http://dx.doi.org/10.1016/j.clay.2009.02.002 [ Links ]
[27] Yeniyol, M. and Lacin, D., IXth National Clay Symposium, Istanbul, Turkey, September, 1999. [ Links ]
[28] Rowell, D.L., Soil Science: Methods and applications, Harlow, UK: Longman Scientific and Technical, 1994, 350 P. [ Links ]
[29] Tyagi, B., C Chudasama,.D. and Jasra, R.V., Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy, Spectrochimica Acta Part A, 64, pp. 273-278, 2006. http://dx.doi.org/10.1016/j.clay.2009.02.001 http://dx.doi.org/10.1016/j.clay.2009.02.002 [ Links ]
[30] McKay, G., Otterburn, M.S. and Aga, J.A., Fuller's earth and fired clay as adsorbent for dyestuffs. Equilibrium and rate studies, Water, Air, and Soil Pollution. 24, pp. 307-322, 1985. http://dx.doi.org/10.1007/BF00161790 [ Links ]
[31] Robertson, R., Fuller's Earth: a History of Calcium Montmorillonite, Volturna Press, 421 P., 1986. [ Links ]
[32] Duran-Rincon, M. y Contreras-C, N., Alternativa de tratamiento para tierra Fuller contaminada con aceite dielectrico, Scientia et Technica, 12 (32), pp. 419-424, 2006. [ Links ]
[33] Agudelo, E.A. y Cardona, S., Análisis preliminar del tratamiento fisicoquímico y biológico del aceite dieléctrico presente en tierra Fuller, DYNA, 78 (167), pp. 193-202, 2011. [ Links ]
[34] Agudelo, E.A., Un método de gestión ambiental adecuado para el tratamiento y la disposición final de un residuo peligroso. Caso: Tierra Fuller contaminada con aceite dieléctrico, Medellín, 2010. [ Links ]
[35] Beltran, O., Berrio, L., Agudelo, E. y Cardona, S., Tecnologías de tratamiento para la tierra Fuller contaminada con aceite dieléctrico, Revista EIA, 10 (19), pp. 33-48, 2013. [ Links ]
[36] Hussin, F., Kheireddine-Aroua, M. and Wan Daud, W.M.A., Textural characteristics, surface chemistry and activation of bleaching earth: A review, Chemical Engineering Journal, 170 (1), pp. 90-106, 2011. http://dx.doi.org/10.1016/j.cej.2011.03.065 [ Links ]
[37] Amado-Duarte, E.S. y Rueda-Gómez, S.L., Aplicación de arcillas bentoníticas modificadas a la adsorción de iones cobre y zinc disueltos en efluentes cianurados, Bucaramanga, 2007. [ Links ]
[38] Ye, H., Zhu, Q. and Du, D., Adsorptive removal of Cd(II) from aqueous solution using natural and modified rice husk, Bioresource Technology, 101, p. 5175--5179, 2010. http://dx.doi.org/10.1016/j.biortech.2010.02.027 [ Links ]
[39] Menéndez, J., Illán-Gómez, M., León C., and Radovic, L., On the difference between the isoelectric point and the point of zero charge of carbons, Carbon, 33 (11), pp. 1655-1659, 1995. http://dx.doi.org/10.1016/0008-6223(95)96817-R [ Links ]
[40] Schoonheydt, R.A. and Johnston, C.T., Chapter 3 Surface and Interface Chemistry of Clay Minerals, in Developments in Clay Science: Handbook of Clay Science, vol. 1, Elsevier, 2006, pp. 87-113. http://dx.doi.org/10.1016/S1572-4352(05)01003-2 [ Links ]
[41] Valenzuela-Díaz, F.R. and De Souza-Santos, P., Studies on the acid activation of Brazilian smectitic clays, Química Nova, 24 (3), pp. 345-353, 2001. http://dx.doi.org/10.1590/S0100-40422001000300011 [ Links ]
[42] Madejová, J., Bujdák, J., Janek, M. and Komadel, P., Comparative FT-IR study of structural modifications during acid treatment of dioctahedral smectites and hectorite, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 54 (10), p. 1397-1406, 1998. http://dx.doi.org/10.1016/S1386-1425(98)00040-7 [ Links ]
[43] Ilic, B.R., Mitrovic, A.A. and Milicic, L.R., Thermal treatment of kaolin clay to obtain metakaolin, Hemijska industrija, 64,(4), pp. 351-356, 2010. [ Links ]
[44] Matocha, C., Clay: Charge properties, in Encyclopedia of Soil Science, Taylor & Francis, 2006. [ Links ]
[45] Önal, M. and Sarikaya, Y., Preparation and characterization of acid-activated bentonite powders, Powder Technology, 172, pp. 14-18, 2007. http://dx.doi.org/10.1016/j.powtec.2006.10.034 [ Links ]
[46] Bajpai, A. and Vishwakarma, N., Adsorption of polyvinylalcohol onto Fuller's earth surfaces, Colloids and Surfaces A: Physicochem. Eng. Aspects, 220, pp. 117-130, 2003. http://dx.doi.org/10.1016/S0927-7757(03)00073-6 [ Links ]
[47] Cardona, S., Reutilización y activación del coque de petróleo para remover metales en agua. Gestión y Ambiente, 9 (1), pp. 89-101, 2006. [ Links ]
O. Beltrán-Pérez, es Ingeniero Químico, MSc. (p) en Aprovechamiento de Recursos Hidráulicos, de la Universidad Nacional de Colombia, Sede Medellín, Colombia.
B. Zuluaga-Díaz, es Ingeniero Químico, de la Universidad Nacional de Colombia Sede Medellín, Colombia.
A. Hormaza-Anaguano, es PhD. en Química. Actualmente es profesora en la Escuela de Química, de la Facultad de Ciencias, Universidad Nacional de Colombia Sede Medellín, Colombia.
S.A. Cardona-Gallo, es PhD. en Ingeniería Ambiental. Actualmente es profesor en la Facultad de Minas, Universidad Nacional de Colombia Sede Medellín, Colombia.