Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.82 no.190 Medellín mar./abr. 2015
https://doi.org/10.15446/dyna.v82n190.46256
DOI: http://dx.doi.org/10.15446/dyna.v82n190.46256
Atmospheric corrosivity in Bogota as a very high-altitude metropolis questions international standards
Corrosividad atmosférica en Bogotá como metrópolis a una gran altitud, inquietudes a normas internacionales
John Fredy Ríos-Rojas a, Diego Escobar-Ocampo b, Edwin Arbey Hernández-García c & Carlos Arroyave d
a Facultad de Ingeniería Mecánica, Universidad Antonio Nariño, Bogotá, Colombia. johnri@uan.edu.co
b Ministerio de Ambiente y Desarrollo Sostenible, Bogotá, Colombia. diescobar@minambiente.gov.co
c Facultad de Comercio Internacional y Economía, Universidad Antonio Nariño, Bogotá, Colombia. edwinh@uan.edu.co
d Vicerrectoría de Ciencia, Tecnología e Innovación, Universidad Antonio Nariño, Bogotá,Colombia. vicerrector.cti@uan.edu.co
Received: October 15th, de 2014. Received in revised form: January 21th, 2015. Accepted: February 6th, 2015
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
This paper presents the first systematic atmospheric corrosion assessment in Bogota. Main facts about the study are related with special characteristics of the City, such as population (more than eight million inhabitants), and altitude (2600 m over the sea level). Relative humidity, temperature, and sulphate dioxide (SO2) concentration were measured. Simultaneously, corrosion rate of AISI-SAE 1006 plain steel was measured throughout a year. Results show that atmospheric corrosion is between low and medium levels, C2-C3, according to the ISO 9223 standard. Nevertheless, estimations from meteorological parameters produce lower corrosivity and, taking into account SO2 concentrations, corrosivity in places with higher relative humidity, are higher than corrosivity measured on steel coupons. In general, the main pollution problem is particulate matter, but higher corrosion rates were directly associated with SO2 levels. Gaps between found results and international estimation methodologies are evident. Some relative explanations are proposed.
Keywords: Brook's Index; ISO 9223 Standard; relative humidity (Rh); time of wetness (TOW); plain carbon steel; atmospheric pollutants.
Resumen
Se presentan resultados del primer estudio sistemático sobre la corrosividad atmosférica de Bogotá, donde se tienen características especiales como una población superior a ocho millones de personas y 2600 m sobre el nivel del mar. Se midieron humedad relativa, temperatura, concentración de dióxido de azufre (SO2) y velocidad de corrosión de acero al carbono AISI/SAE 1006. La corrosividad encontrada se ubica entre los niveles bajo y medio, C2-C3, según la norma ISO 9223. No obstante, los valores estimados a partir de los parámetros meteorológicos dan resultados menores y, de acuerdo a la concentración del SO2, las corrosividades en los sitios con mayor humedad relativa son mayores que las medidas en platinas de acero. El principal problema de contaminación es material particulado, pero las mayores tasas de corrosión estuvieron asociadas con los niveles de SO2. Diferencias entre los valores medidos y estimados son evidentes, proponiendo algunas explicaciones acerca de ello.
Palabras claves: Índice de Brooks; norma ISO 9223; humedad relativa; tiempo de humectación; acero de bajo carbono; contaminantes atmosféricos.
1. Introduction
The stability of materials used in building the infrastructure that supports the development of society is one of the big challenges of engineering since aspects such as health and people's safety need to be assured. Also necessary is the optimization of the use of resources (their quantity, costs, maintenance requirements and necessary replacements). In this sense, one the most significant problems is the stability of materials exposed to the atmosphere of big cities, where pollutants are normally found. Pollutants tend to accelerate the deterioration of different types of materials used to build cities, such as metals, ceramics, polymers or combinations of these materials. In this respect, each city and each place has particular characteristics that can influence the loss of material properties. Studies on atmospheric corrosion performed in many cities have allowed for the conclusion that the nature of atmospheric pollutants and their level of concentration play an important role in the service life of different engineering materials [1-3].
Bogota's natural barriers are the mountains in the east of the City and the Bogota River in the west. Bogota is characterized by having a bimodal rainfall and a temperature range of between 7 and 18°C [4]. The city extends around 380 km2 and hosts a population estimated at more than eight million inhabitants, with a growth rate that places it in sixth place among the big cities in the world. The greatest growth rate is estimated for between 2010 and 2025 [5]. The average elevation of the city is 2600 meters above sea level. Such elevation provides the city with some particular characteristics compared to previous studies in other latitudes. Additionally, no systematic studies on loss of integrity of the materials exposed to the atmosphere have been undertaken in Bogota. The quantity of structural materials used in urban infrastructure and their direct interaction with the atmosphere that may cause significant aggresivity (average temperature values (T) and relative humidity (Rh) are 14°C and 70%, respectively; along with the concerning atmospheric pollution) lead to one thinking beforehand that there is significant deterioration of the materials used to build the City.
To make it clear, costs caused by corrosion, including material deterioration, maintenance, replacement, problems generated because of outages and delays, penalties, as well as prevention and control measures are really significant. It has been established that direct costs generated by corrosion in a country, account for five percent of the GDP. Additionally, due to the fact that around 80 per cent of the materials are exposed to the atmosphere, costs of atmospheric corrosion can be 30 to 50 percent of the total costs of corrosion. As a consequence, in the case of Bogota, with a high urban concentration, it can be estimated that direct costs annually generated by atmospheric corrosion can be around two to three percent of its GDP (It was USD $92.917 million in 2012 - [6]), which would mean costs generated by atmospheric corrosion, for the same year, were USD $1.858 and USD $2.788 million).
Weather plays an important role when assessing atmospheric aggression in a particular area. Factors such as T, Rh, precipitation and air pollution, among others, can determine the magnitude of atmospheric corrosion of metallic elements exposed to such conditions [7]. The meteorological parameters that are most associated with atmospheric corrosion are T and Rh. Based on chemistry principles, it is known that an increase in T tends to stimulate the attack since there is a speedy increase of electrochemical reactions and diffusion processes. However, taking into account the electrochemical mechanisms of atmospheric corrosion, the same increase of T can contribute to the reduction of the humidity layer on the surface of the material. As a consequence, there is a reduction in the attack; thus, the process turns into something very complex since many factors-which act simultaneously, sometimes stimulating, sometimes inhibiting the attack process-can be identified. Among those factors, some pollutants, which tend to significantly accelerate the attack, and thus, have led to several studies, can be highlighted; in particular, the presence of sulfur dioxide (SO2) in urban and industrial atmospheres and chlorides in areas influenced by the sea [8].
A great number of variables that intervene in weather conditions make corrosive assessment of the atmosphere a complex issue, which is difficult to measure. In real life, different approaches have been used to estimate the behavior of a material in a given atmosphere. These include: a) direct measurement of atmospheric corrosion through detailed follow up of simple behaviors of materials of interest; b) estimation of atmospheric corrosion from environmental data and corrosivity experimentation; and c) application of dose-response functions or damage function, which have been found to be associated with the degree of deterioration of similar materials in equivalent microclimates [9,10].
To this end, some methodologies have been developed; they aim at facilitating estimations, and, as a consequence, foreseeing behaviors. These are: i) calculation of Brooks' index, which estimates the degree of deterioration taken from the Rh and T average annual values [11]; ii) the application of the ISO 9223 standard, based on environmental parameters and field sample exposure (ISO 9223) and, iii) through the application of a function to predict annual damage caused by atmospheric corrosion. Details of these methodologies will be presented below.
1.1. Brooks' Index of atmospheric aggressiveness
The equation that allows for the determination of the Brooks' Deterioration index relates the potential risk of atmospheric corrosion correlated to the deterioration index (I), which is determined through the eq. (1) [11]:

Rh is the average relative humidity and Pv is the saturation pressure of vapor at average temperature expressed in mbar, given that Rh is the quotient between the quantity of water vapor present in the air and the maximum quantity of vapor that may be present in air for specific T and pressure (P).
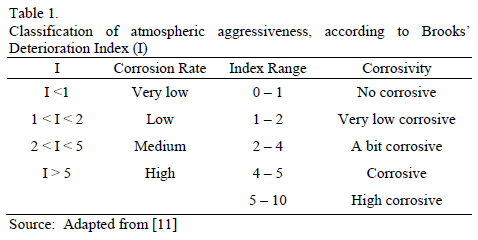
Once the average annual values of Rh and T are obtained, the Pv values are estimated to determine the deterioration index (I) through eq. (1). The values obtained allow us to make approximations of the corrosion rate of metallic materials exposed to such conditions and, additionally, scoring the degree of corrosion in the atmosphere assessed, as shown in Table 1.
Without a doubt, this methodology aims at indirectly estimating the impact of the thickness of the electrolyte layer built on the surface of the material. There, the electrochemical phenomenon of metal atmospheric corrosion is developed to a greater or lesser extent. Effects such as those held by microclimatic factors, i.e. atmospheric pollutant concentration with potential impact on nature and the extension of reactions happening within the electrolyte are not being taken into consideration.
1.2. Atmospheric corrosivity according to ISO 9223
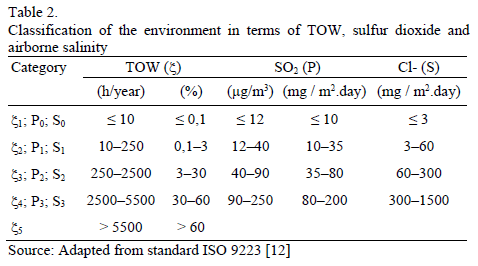
The international standard ISO 9223 [12] is a more realistic approximation, which takes into account the presence of the most relevant atmospheric pollutants, and uses meteorological and environmental parameters, as well as direct measurement of the corrosion rate of test specimens. Thus, it is possible to determine atmospheric corrosion in a specific place by calculating the time of wetness (TOW), and level of deposition of SO2 and chlorides (Cl-). TOW refers to the time in which a metallic surface presents a water layer sufficiently thick for the electrolyte to act, thus, corrosion takes place. Such time is estimated as the yearly number of hours in which Rh exceeds 80% and T has been above 0°C. The categories presented in Table 2 are established using TOW and average levels of SO2 and Cl.
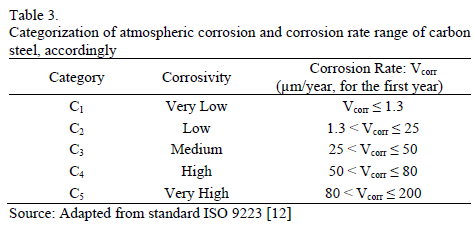
Once time of wetness and pollutant categorization has been performed, atmosphere is classified from the point of view of corrosiveness, based on the tables available in the ISO 9223 standard for some base materials. It is then associated to the five attack rate categories. Regarding steel, for instance, the corrosion rate range is shown in Table 3.
When one has corrosion rate values, directly measured on carbon steel specimens, categorization of atmospheric corrosion is established from the range where the data obtained is located.
1.3. Estimations according to a corrosion damage function
A great number of damage functions or dose-response equations, which are compared to the corrosion of some materials with environmental parameters, have been determined in several studies. Among some of the most significant results worth mentioning is the establishment of an equation from data resulting from corrosion of carbon steel, obtained in a great number of areas, which are distributed in different countries in order to apply it globally [13]; or dose-response functions that are aimed at collecting the impact of a significant number of pollutants simultaneously [14], and dose-response functions that have been established to set international standards such as ISO.
One of the most widely recognized results is eq. (2) [1],
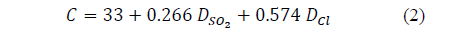
Where: C stands for the corrosion rate for carbon steel, in mm.yr-1. DSO2 is the annual average SO2 deposition in mg SO2.m-2.d-1 and DCl is the annual average of chloride deposition in mg Cl.m-2.d-1. In this sense, the attack rate of metal in the atmosphere can be determined by knowing the pollutant concentration. This equation is similar to others that have been developed to achieve global validity. [13].
On the other hand, the dose-response function presented as eq. (3) compares the annual corrosion rate of carbon steel in mm.yr-1, using values of T, Rh, SO2 and Cl-, without taking into account TOW, and it was developed to improve the ISO classification system [15]:
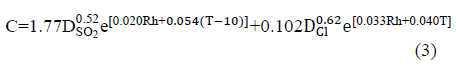
1.4. Field tests
Determination of the corrosion rate of carbon steel can be carried out directly through exposure of samples of such material in the specific points of interest. In order to carry this out, structures called exposure racks are located according to the ASTM G-50 [16] standard, which specifies position, and height for locating the test specimens and other details related to the location of samples. Subsequently, the structures are removed after certain exposure times, with differences of months in the beginning and normally years at the end.
As far as we know, no research has been undertaken that performs a systematic analysis of the atmospheric corrosion problems in Bogota and, even less so to analyze different behavior in different parts of the city which can be classified according to microclimates identified based on meteorological and pollutant concentration data that have been collected over several years. Obviously, it was of great importance to collect relevant information and analyze it according to internationally recommended procedures to identify how atmospheric corrosion is distributed throughout the City. Subsequently, such information can be used for design, where it is very important to estimate the life service of structures, optimize the choice of materials to use or in decision making when a specific material requires protection from a corrosive attack.
Therefore, the current study was aimed at assessing general atmospheric corrosion conditions for Bogota city, applying Brooks' indexes methodology and determining risk of metallic material deterioration, following the methodology established by ISO 9223 standard, through application of damage expressions of annual atmospheric corrosion and, determination of corrosion rated of standard specimens.
2. Methods and materials
2.1. Meteorological and atmospheric pollution data
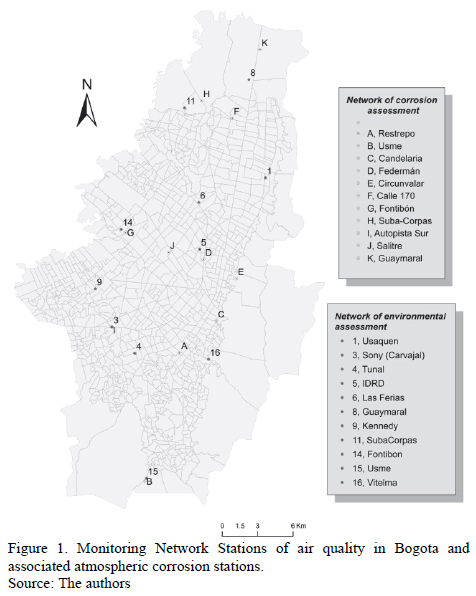
Average data for Rh, T and [SO2] were taken from the records captured every hour in different network stations monitoring the quality of air in the city from 2008 to 2012. Fig. 1 shows the locations of monitoring stations distributed throughout Bogota, according to the recommendations of international standards on the subject [17]. Two stations only worked half the time, and others did not record all parameters.
SO2 average annual concentration values received as ppb were converted into µg/m3, using the eq. (4):

Where ppb corresponds to the [SO2] value reported by the network, PM is SO2 molecular weight, and the constant 31.98 corresponds to molar volume of an ideal gas for T and P average conditions in Bogota.
Since Bogota is a completely Mediterranean city, more than 1000 km away from any sea, Cl- concentration data is not usually recorded. Nevertheless, there are recent reports that present a deposition of less than 3 mg.m-2.d-1 [10,8]; therefore, in this paper, it is assumed that chloride concentration does not represent a significant value.
2.2. Materials
AISI/SAE 1006 Carbon steel plates (0.061% C, 0.008% S, 0.180% Mn, 0.019% Cu, 0.040% Al), of 100 mm x 150 mm, previously degreased, rinsed with alcohol, dried with hot air and weighed, were exposed in triplicate in each site for a year beginning in the first six months of 2012 and ending in the first six months of 2013. Samples were exposed at atmospheric corrosion stations, which were built following the guidelines of ASTM G50 standard and located in different sites of the city. Location of the test specimens was defined following recommendations of standards for sampling of total sulfating activity, as well as criteria of network design for monitoring air quality. [17,19]. Subsequently, after exposure, samples were taken to the laboratory to determine loss of mass, after chemical cleaning procedure. [20]
2.3. Exposure sites
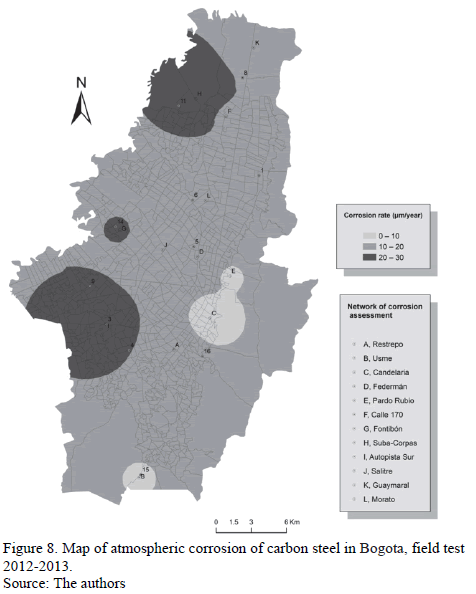
Exposure sites were chosen taking into account climatological data reported by the City's monitoring network. In general, it was sought to install corrosive stations near the environmental network stations, which were characteristic of microclimates of interest. Distribution of measurement corrosion sites was: two stations in the north (N), one in the Northwest (NW), two in the Eastern Center (EC), three in the Southwest (SW) and three in the South (S). Location sites of these atmospheric corrosion stations are shown in Fig. 1.
3. Results and discussion
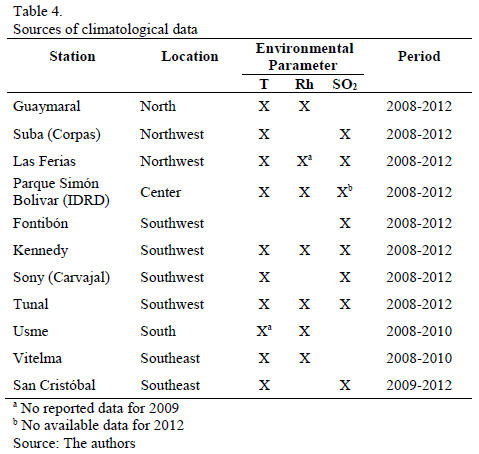
Table 4 presents a list of environmental monitoring network stations, which were used as reference for locating corrosion stations and, therefore, provided sources for climatological data, associable to corrosion data. Parameters recorded and periods in which those parameters were measured are detailed in the Table as well.
3.1. Climatological data of reference
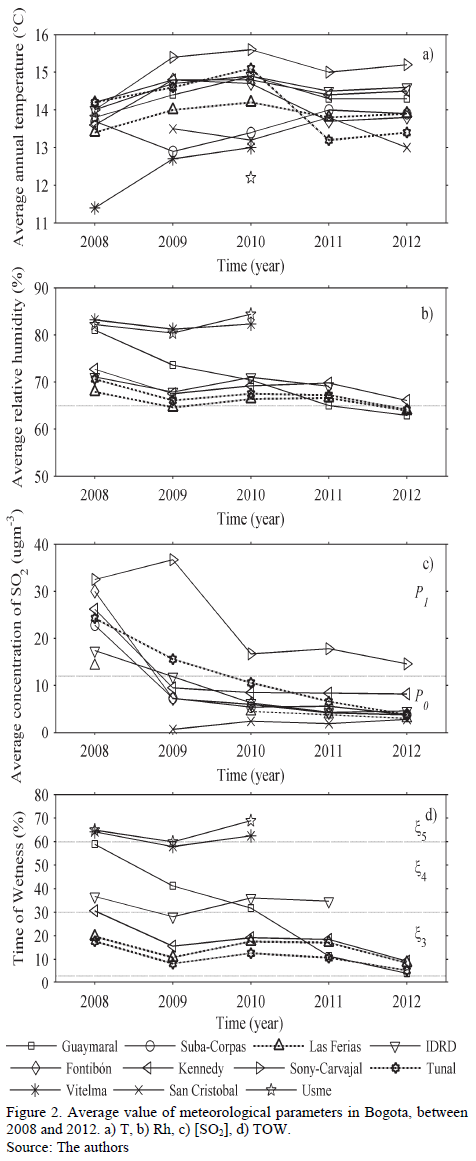
Annual average T and Rh measured in reference stations are detailed in Figs. 2a, 2b. Annual average T observed in different sites of the city is between 11 and 16°C, presenting the lowest values in the south of the city. Correspondingly, Rh is higher in these zones; a similar situation is shown with this latter variable, in the Northern zone (Guaymaral Station), during 2008 and 2009.
The SO2 average annual concentration is shown in Fig. 2c, which indicates that values have been declining substantially during the last few years in almost all of the sites (global trend in most cities thanks to worldwide efforts to minimize emission of pollutants from fossil fuels). Additionally, the highest pollution was present in the Southwest zone (Carvajal station, followed by Kennedy station). The other stations report very low [SO2] over the last few years. Thus, SO2 volumetric concentration in Bogota corresponds to low or minimum levels, which would allow for classifying its atmosphere in categories ISO P0 and P1.
Fig. 2d is a histogram of TOW evolution estimated for each station, per year. In general, this parameter is located in category ISO x3, except for Parque Simón Bolívar station (great green lung in the central area of the city) located in category ISO x4, and stations Usme and Vitelma located in category ISO x5, both associated to rural or semirural microclimates. There are still no satisfactory explanations about time of wetness reduction in Guaymaral station.
3.2. Relationship between Rh and TOW
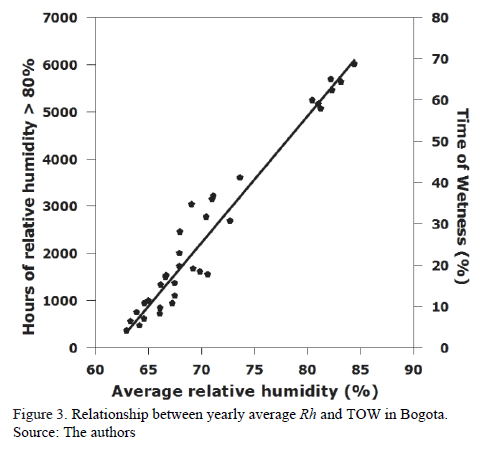
Average annual Rh and annual hours in which the value of said parameter was higher than 80% (which allows for deducting TOW indirectly)-obtained in an established time window (2008-2012)-present good lineal correlation. Fig. 3 illustrates this relation. It can be said, and suggested in the literature [21], that in Bogota for instance, TOW can be deduced directly from average annual Rh.
The model proposed to estimate the relation is represented in Eq. (5):
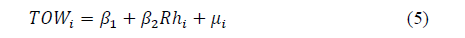
According with the model, eq. (6) and (7) were estimated for determining the relationship between the Time Of Wetness and Humidity Relative in Bogota:
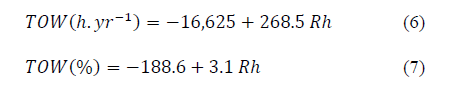
It is important to point out that there is an optimum fit for both models. In both cases, R2 is approximately equal to 95%. Both the model and individual parameters are globally relevant for any level of significance. This means that the null hypothesis, when the coefficients are statistically equal to zero, are rejected and therefore the estimated model is good [22].
3.3. Corrosivity estimation from meteorological parameters: Brooks Index
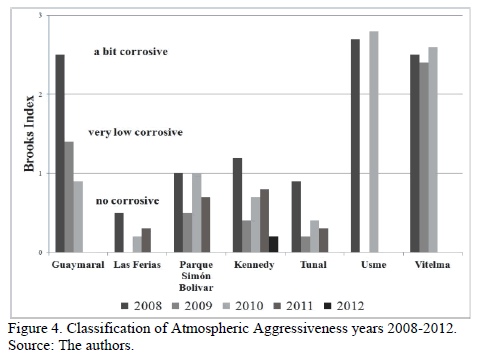
Deterioration indexes in eight stations are obtained by taking data from Fig. 3 and using eq. (1): Guaymaral, Las Ferias, Parque Simón Bolívar, Kennedy, Tunal, Usme and Vitelma. Deterioration indexes obtained for each station and for each study period, as well as corresponding classification are shown in Fig. 4.
Results obtained according to Brooks' equation become the first approximation of potential risks of atmospheric corrosion in Bogota city, compared to weather conditions described exclusively through Rh and Pv variables assuming a corrosive pollutant free atmosphere.
It can be said, according to the values obtained (Fig. 4) that in 2008 conditions of higher aggressiveness took place since, in general, Rh values were higher.
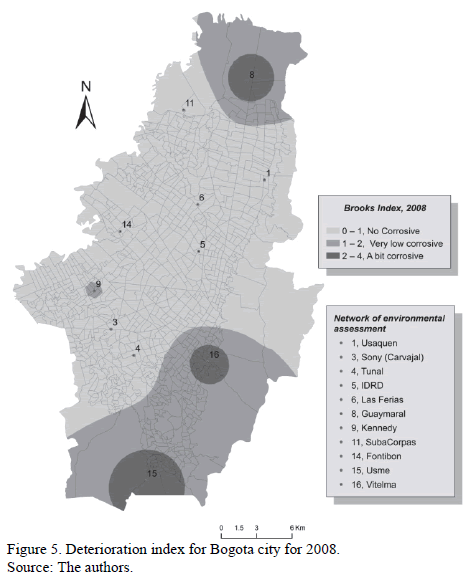
The Map in Fig. 5 presents, comprehensively and synthetically, all the data associated to aggressiveness of different microclimates according to Brooks Indexes for 2008. The building of the map as well as management and handling of geographic data was performed using Arcmap 10 tool of the Geoestatistical Analyst software.
The map allows us to score the Usme, Ciudad Bolívar, Suba and Usaquén microclimates as the most aggressive. Nevertheless, Usme along with Guaymaral and Vitelma can be identified as little aggressive while Tunal and Las Ferias can be identified as non-aggressive.
In the subsequent years (Fig. 4), Vitelma and Usme stations kept their little aggressive nature while the rest have shown a tendency to become non-aggressive.
Finally, it can be said that Brooks' Indexes associated to corrosive and high corrosive categories were not present in any of the cases. Therefore, it can be deduced that from an Rh perspective, Bogota's atmosphere does not present conditions that can be considered highly corrosive.
3.4. Atmospheric corrosivity according to pollutant concentrations: ISO standard 9223
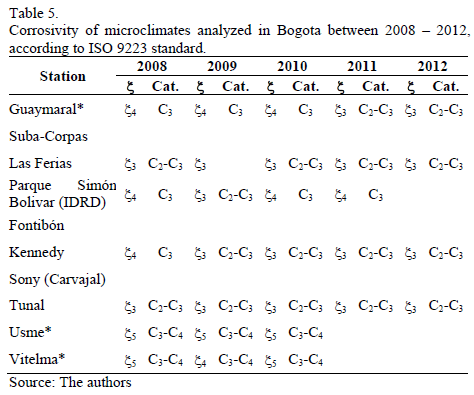
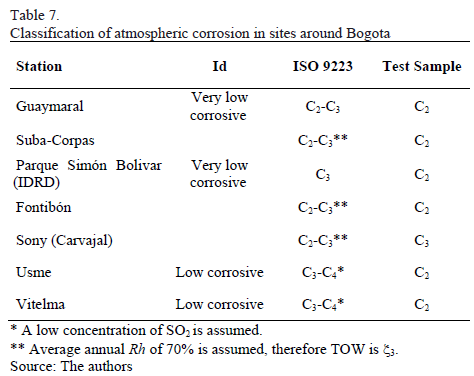
As it has already been stated, a way to estimate atmospheric aggressiveness takes into account TOW and SO2 and Cl- concentrations; the latter is not significant in the case of Bogota. Likewise, it is reiterated that the average concentration of SO2 in Bogota is low since it has always shown annual values lower than 40 mg.m-3. On the other hand, according to Table 2, it can be inferred that wetting percentage can be associated principally to categories 3 and 4. Therefore, and taking into account the ISO 9223 standard, it can be concluded that Bogota's atmosphere exhibits corrosivity of carbon steel which can be classified between low and average (C2 - C3). In other words, typical corrosion rates fluctuate between 1.3 and 50 mm.yr-1. Additionally, particularly in the Usme and Vitelma stations, with higher Rh values and, therefore higher values in TOW, greater corrosion is determined associated to categories C3-C4, which, according to Table 2, correspond to corrosion rates of between 25 and 80 mm.yr-1. A set of results from the different stations taken into account is collected in Table 5.
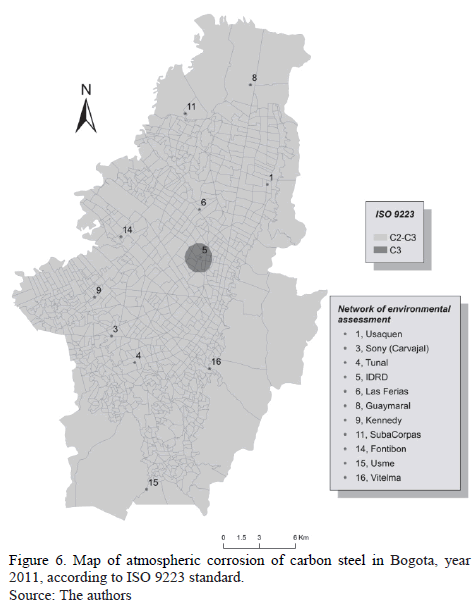
A map of atmospheric corrosion in Bogota, shown in Fig. 6, was built using the same data estimated by the application of the ISO 9223 standard for 2011.
The following fact is reconfirmed: in general, aggressiveness is classified as mild-in the C2-C3 range, with some emphasis on the upper limit in the center of the City-and it can be associated to higher TOW.
3.5. Corrosion rates according to dose-response functions.
The SO2 Volumetric concentration data (mg.m-3) reported by the monitoring network was converted to deposition rates (mg of SO2.m-2.d-1), in such a way that the carbon steel corrosion rate could be estimated through eq. (2). Such conversion was undertaken using eq. (8) [12]:

As it has already been stated, given the atmospheric conditions being studied, reconfirmed with some previous baseline data, it was feasible to disregard any possible impacts of chloride ions.
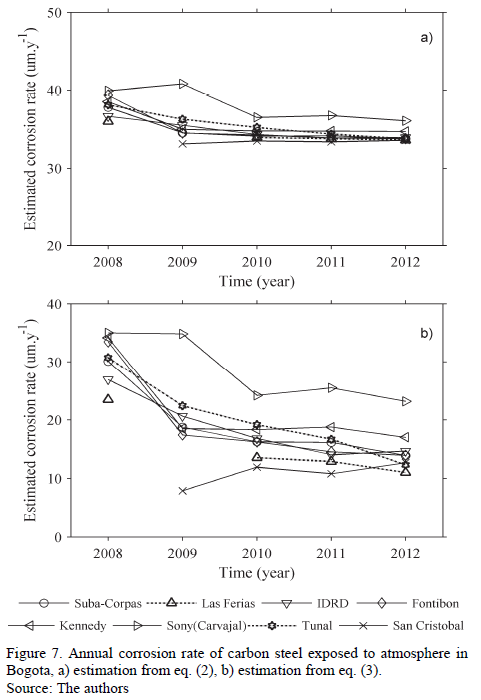
In Fig. 7a, corrosion rates estimated during the years taken into account were compensated. In Fig. 7a, a tendency of reduction in corrosion rate is shown between 2008 and 2010, followed by relative stabilization. Obviously, since these rates depend directly on [SO2], Fig. 7a and 2c are analog. Regarding the significance of the corrosion rate, always above 33 mm.yr-1, it is important to remember a constant impact in eq. (2), which does not allow for observing direct proportionality between pollutant concentration and deterioration grade at low [SO2].
As an alternative, eq. (3) can be applied. In the cases where actual Rh data was not available, 70% was assumed to make the calculations, since that is the average recorded value with high frequency during the last few years in several zones of the City. Resulting corrosion rates were compensated in time and represented in Fig. 7b.
These last calculations support the trend of a reduction in aggressiveness in the 2008-2010 triennium, and the relative stabilization in the subsequent period. Additionally, Carvajal and Kennedy Southwestern stations, which correspond to microclimates of high industrial activity, stand out due to their higher corrosion rates. In any case, corrosion rates can still be classified as mild, in category C2 to C3, according to ISO classification. As a consequence, it can be stated that in measuring aggressiveness in Bogota's atmosphere, the Brooks Index methodology and ISO methodology give equivalent results and suggest that, in general, there are microclimates with corrosion that goes from low to medium.
3.6. Direct corrosion rate measurements
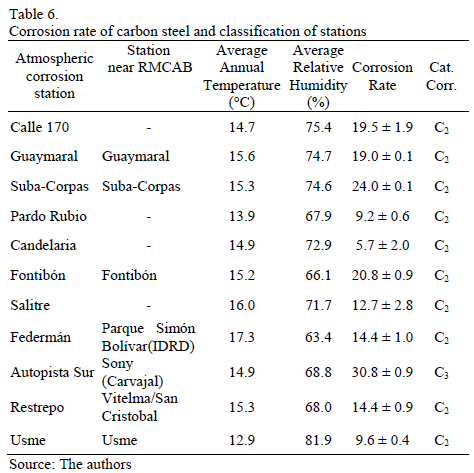
The corrosion rate values of carbon steel test specimens which were exposed in the different study sites for a year are presented in Table 6. The map of the atmospheric corrosion of carbon steel in Bogota derived from these data is shown in Fig. 8.
As we can see in Table 6, the corrosion rates of carbon steel, during the first year of exposure in different microclimates in Bogota, are lower than 32 mm.yr-1. This proves that, in fact, aggressiveness in the city's atmosphere is low or medium. Only Carvajal station, with an industrial microclimate and evidently higher [SO2], presents corrosion rates associated to category C3. The predominant role of SO2 as the main atmospheric pollutant is reconfirmed from the point of view of material stability, in non-marine environments [23].
In the case of Suba, Fontibón and Autopista Sur stations, it is interesting to observe that the annual rates of atmospheric corrosion differ only between 25 and 40 percent compared to values estimated when applying eq. (3). However, corrosion rates measured in Federmán and Restrepo stations show differences lower than 15 percent compared to rates calculated with the same eq. (3). A striking difference between the two groups of stations is associated to the strong differences in the homogeneity of the environment of the first three stations because of the assumed value Rh for the first three stations and the homogeneity of the environment in climatological and corrosion stations of the last two stations.
On the other hand, Usme and Pardo Rubio stations, with the lowest pollution levels, show very low corrosion rates. In the first station, the rural nature of the area is reaffirmed. In the case of Pardo Rubio, the impact of a particular topography is clear. Despite being inside the city, the station is located on top of a mountain, delimiting its Eastern edge and 150m above the rest of the city. In those stations, their corrosivity, type C2, is lower than the corrosivity estimated from environmental parameters. Therefore, the limitation of ISO 9223 standard, which has been also identified by other authors [13, 24], is reaffirmed in cases whereby even if there are high Rh and high TOW, pollution levels are low.
To conclude, atmospheric corrosion of carbon steel in the city corresponds to low-to-medium corrosivity, according to the results presented above and listed in table 7 using three different methodologies.
Estimations made using Brooks aggressiveness index and ISO standard, in terms of wetting and pollutant levels, allow us to obtain approximations of corrosiveness. However, estimations can be undervalued when they are performed in areas of high relative humidity, such as in the case of Usme and Vitelma stations. This was stated above.
4. Conclusions
The reported results correspond to the first systematic study about atmospheric deterioration of materials exposed in Bogota's atmosphere. Some of the combined peculiarities, mainly high anthropogenic activity, plus high altitude (closely related with meteorological parameters such as solar radiation and relative humidity) influence the behavior of materials and, consequently, expected standard deterioration rates are not obtained.
In this sense, corrosion rates estimated according to Brooks' Index are lower than field measurements. Furthermore, atmospheric corrosivity estimated according to the ISO 9223 standard, in sites with low pollution levels and high relative humidity, are higher than the measured ones. Both cases could be related to the deviation of the standards, due to the atmospheric peculiarities.
An average atmospheric corrosivity on plain carbon steel, in Bogota, could range between low and medium levels. The highest values are usually associated with higher SO2 levels, and closely to the higher population and industrial plant concentrations. However, factors such as the city's geometry could be another influencing factor.
There is a good correlation between the measured corrosion rates and the dose-response function proposed by Mikhailov. Consequently, this equation is proposed as a first step to approach any estimation about life expectation on plain carbon steel structures used in Bogota.
Low concentrations of well-known pollutants such as SO2 are not sufficient guarantee of stability for steel or any other material exposed to an atmosphere like the one studied here. Many other negligible pollutants could be taking part in complex chemical reactions happening on material surfaces.
Acknowledgements
The authors acknowledge Antonio Nariño University for the support given for the development of project No. 2010257. Likewise, the authors express their gratitude to Secretaría de Ambiente de Bogota (Bogota Environmental Protection Agency) for providing meteorological data and pollution levels used in this paper. The authors also acknowledge the following institutions and companies for allowing them to locate stations in their facilities: Hilandería Fontibón, Almacenes MAKRO, Hyundai de Colombia, Museo Interactivo MALOKA and CIEDI School.
References
[1] Feliu, S., Morcillo, M. and Feliu Jr, S. The prediction of atmospheric corrosion from meteorological and pollution parameters - I. Annual corrosion. Corrosion Science, 34 (3), pp. 403-414, 1993. http://dx.doi.org/10.1016/0010-938X(93)90112-T [ Links ]
[2] Kucera, V. Reduction of air pollutants - A tool for control of atmospheric corrosion. Revista de Metalurgia (Madrid), (SPEC.VOLUME), pp. 55-61, 2003. http://dx.doi.org/10.3989/revmetalm.2003.v39.iExtra.1097 [ Links ]
[3] De la Fuente, D., Vega, J.M., Viejo, F., Díaz, I. and Morcillo, M. Mapping air pollution effects on atmospheric degradation of cultural heritage. Journal of Cultural Heritage, 14 (2), pp. 138-145, 2013. http://dx.doi.org/10.1016/j.culher.2012.05.002 [ Links ]
[4] Vargas, A., Santos, A., Cárdenas, E. and Obregón N. Análisis de la distribución e interpolación espacial de las lluvias en Bogotá, Colombia. Revista DYNA, 78 (167), pp. 151-159. 2011. [ Links ]
[5] N.A. The century of the city. Nature, 467, pp. 900-901, 2010. DOI: 10.1038/467900a [ Links ]
[6] Pineda, S., et. al. Ranking de Ciudades Latinoamericanas para la Atracción de Inversiones, Informe Oficial. Bogotá, Universidad del Rosario - Inteligencia de Negocios, 2012, 49 pp. [ Links ]
[7] Oesch, S. The effect of SO2, NO2, NO and O3 on the corrosion of unalloyed carbon steel and weathering steel - the results of laboratory exposures. Corrosion Science, 38, pp. 1357-1368, 1996. http://dx.doi.org/10.1016/0010-938X(96)00025-X [ Links ]
[8] Mendoza, A.R. and Corvo, F., Outdoor and indoor atmospheric corrosion of carbon steel. Corrosion Science, 41 (1), pp. 75-86, 1999. http://dx.doi.org/10.1016/S0010-938X(98)00081-X [ Links ]
[9] Johan Tidblad, Vladimir Kucera, Alexandre A. Mikhailov, Jan Henriksen, Katerina Kreislova, Tim Yates, Bruno Stöckle and Manfred Schreiner. UN ECE ICP Materials: Dose-response functions on dry and wet acid deposition effects after 8 years of exposure. Water, Air, and Soil Pollution, 130(1-4), pp. 1457-1462, 2001. http://dx.doi.org/10.1023/a:1013965030909 [ Links ]
[10] Botero Vega, C.A. Evaluación de la corrosividad de atmósferas colombianas y su impacto sobre el deterioro de algunos materiales empleados en el sector eléctrico, Medellin, Universidad de Antioquia, 2008, pp. 166. [ Links ]
[11] Brooks, C.E.P., Climate in everyday life, Dent, Londres, 1950. [ Links ]
[12] ISO 9223, Corrosion of metals and alloys, Corrosivity of atmospheres, Classification, ISO, Geneve, Switzerland, pp. 14, 1992. [ Links ]
[13] Morcillo, M., Almeida, M.E., M., R.B., Uruchurtu, J. and Marrocos, M., Corrosión y Protección de Metales en las Atmósferas de Iberoamérica. Parte I: Mapa Iberoamericano de Corrosividad Atmosférica, Madrid, 1999. [ Links ]
[14] Tidblad, J., Kucera, V. and Mikhailov, A.A., Statistical analysis of 8 year materials exposure and acceptable deterioration and pollution levels, INSTITUTE, S.C., 1998, pp. 49. [ Links ]
[15] Mikhailov, A.A., Tidblad, J. and Kucera, V. The classification system of ISO 9223 standard and the dose-response functions assessing the corrosivity of outdoor atmospheres. Protection of Metals, 40 pp. 541-550, 2004. http://dx.doi.org/10.1023/B:PROM.0000049517.14101.68 [ Links ]
[16] ASTM G50, Standard Practice for Conducting Atmospheric Corrosion Tests on Metals, American Society for Testing and Materials, West Conshohocken, United States, pp. 5, 2003. [ Links ]
[17] EPA. Revisions to Ambient Air Monitoring Regulations, Final rule. Environmental Protection Agency, Federal Register, 71 (200) pp. 61236-61328, 2006. [Consulta, march 21 of 2011]. Available at: http://www.gpo.gov/fdsys/pkg/FR-2006-10-17/pdf/06-8478.pdf [ Links ]
[18] Castaño, J.G., Botero, C.A., Restrepo, A.H., Agudelo, E.A., Correa, E. and Echeverría, F. Atmospheric corrosion of carbon steel in Colombia. Corrosion Science, 52 (1), pp. 216-223, 2010. http://dx.doi.org/10.1016/j.corsci.2009.09.006 [ Links ]
[19] ASTM D2010, Standard Test Methods for Evaluation of Total Sulfation Activity in the Atmosphere by the Lead Dioxide Technique, American Society for Testing and Materials, Pensylvania, United States, pp. 2004. [ Links ]
[20] ASTM G1, Standard Practice for Preparing, Cleaning, and Evaluation Corrosion Test Specimens, American Society for Testing and Materials, Pensylvania, United States, pp. 5, 1999. [ Links ]
[21] Feliu, S. and Morcillo, M. La meteorología en España. Análisis de los principales parámetros meteorológicos con influencia en los fenómenos de corrosión, en Feliu, S. y Morcillo, M., Mapas de España de corrosividad atmosférica, Madrid, Morcillo, M y Feliu, S, 1993, pp. 11-30. [ Links ]
[22] Gujarati, D.N. and Porter, D.C., Basic econometrics, Fifth edition, McGraw-Hill, 2009, pp. 946. [ Links ]
[23] Matsushima, I. Carbon Steel - Atmospheric Corrosion, in R. Winston Revie (Editor), Uhlig's Corrosion Handbook, 2nd edition, New Jersey, John Wiley & Sons, Inc., 2000, pp. 515-528. [ Links ]
[24] Santana Rodríguez, J.J., Santana Hernández, F.J. and González González, J.E. The effect of environmental and meteorological variables on atmospheric corrosion of carbon steel, copper, zinc and aluminium in a limited geographic zone with different types of environment. Corrosion Science, 45, pp. 799-815, 2003. http://dx.doi.org/10.1016/S0010-938X(02)00081-1 [ Links ]
J. F. Ríos-Rojas graduated as a Chemical Engineer in 2004 and completed his PhD degree in Engineering in 2012, both from the Universidad de Antioquia, in Medellín - Colombia. Currently, he is an Assistant Professor in the Mechanical Engineering School and researcher in the Group of Research in Energy and Materials at Universidad Antonio Nariño (Bogotá - Colombia). His main subjects of interests in R&D are atmospheric corrosion, aqueous corrosion and protective coatings.
D. Escobar-Ocampo, Environmental and Sanitary Engineer from La Salle University, Specialist in Geographic Information Systems from the Francisco José de Caldas University and candidate to a master in Engineering from the National University of Colombia. Currently serves as a specialized professional with the chemicals, hazardous waste and ozone technical unit (OTU) at the Environment and Development Ministry in Colombia.
E. A. Hernández-Garcia, graduated as an Economist and is candidate to a Master of Science Applied Mathematics, both from the National University in Bogotá - Colombia. Since 2010, he has been an Assistant Professor and researcher at the Universidad Antonio Nariño.
C. Arroyave graduated as a Metallurgical Engineer from the Universidad de Antioquia, in Medellin (Colombia, 1979). He obtained a M.Sc. in Metallurgical Engineering and Materials Science (1989) from the Universidade Federal do Rio de Janeiro (Brazil); and has a Ph.D. in Chemical Science (1995) from the Universidad Complutense de Madrid (Spain). He spent a postdoctoral stay from 2001 to 2002 at the Swedish Corrosion Institute. He was member of the faculty of the Department of Metallurgical Engineering from 1979 to 2008, and Dean of the School of Engineering of the Universidad de Antioquia, from 2004 to 2007. He was distinguished as Titular and Emeritus Professor of the Universidad de Antioquia. Since 2010, he has been the Vice-Chancellor for Science, Technology and Innovation of the Antonio Nariño University. His main subjects of interests in R&D are atmospheric corrosion, protective coatings, weathering steels, and iron oxides.