Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.82 no.193 Medellín set./out. 2015
https://doi.org/10.15446/dyna.v82n193.44217
DOI: http://dx.doi.org/10.15446/dyna.v82n193.44217
A comparative study of two aging and fusion methods in the synthesis of zeolitic materials from natural clinker
Estudio comparativo de dos métodos de envejecimiento y fusión en la síntesis de materiales zeolíticos a partir de clinker natural
Jeimer Alexander Lizcano-Cabeza a, Luis Fernando Ávila-Ascanio a,Carlos Alberto Ríos-Reyes b & Luz Yolanda Vargas-Fiallo a
a Escuela de Química, Universidad Industrial de Santander, Bucaramanga, Colombia, jalc91@hotmail.com
a Escuela de Química, Universidad Industrial de Santander, Bucaramanga, Colombia, luisferavi@hotmail.com
a Escuela de Química, Universidad Industrial de Santander, Bucaramanga, Colombia, levargas@uis.edu.co
b Escuela de Geología, Universidad Industrial de Santander, Bucaramanga, Colombia, carios@uis.edu.co
Received: June 30th, 2014. Received in revised form: May 5th, 2015. Accepted: July 28th, 2015.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract
A sample of Colombian natural clinker was evaluated as a raw material in a laboratory-scale synthesis of zeolitic materials using two methods of alkaline fusion, followed by two aging methods, and finally hydrothermal treatment during three different periods of time. Fusion was carried out with solid and aqueous NaOH and followed by static or ultrasonic aging. The process ended with a hydrothermal reaction. Natural clinker was converted into several zeolitic materials. The characteristics of the resulting zeolitic materials and the effects of experimental variations are discussed.
Keywords: natural clinker; aging method; synthesis; zeolitic materials; fusion.
Resumen
Una muestra de clínker natural colombiano fue evaluado como material de partida para una síntesis a escala de laboratorio de materiales zeolíticos usando dos métodos de fusión alcalina seguida de dos métodos de envejecimiento y finalmente un tratamiento hidrotérmico bajo tres periodos de tiempo distintos. La fusión se llevó a cabo usando NaOH sólido y en solución, siendo seguida por envejecimiento en condiciones estáticas o de ultrasonido. El proceso finalizó con una reacción hidrotérmica. El clínker natural fue convertido en varios materiales zeolíticos. Las características de los materiales zeolíticos resultantes y los efectos de las variaciones experimentales son discutidos.
Palabras clave: clínker natural; método de envejecimiento; síntesis; materiales zeolíticos; fusión.
1. Introduction
Zeolite synthesis from coal by-products like natural clinker (NC) using several methods has recently been receiving a lot of attention. Since the pioneering works of Höller and Barth-Wirsching [1] and Ríos et al. [2] who were the first researchers to have synthesized zeolites from fly ashes and natural clinker respectively, much investigation has been conducted to convert these coal by-products to zeolites. The conversion of coal by-products into zeolites can be conventionally developed by hydrothermal crystallization under alkaline conditions, which has been reported by different authors [1-16]. Recently, the conventional alkaline conversion of these materials has been improved by using more sophisticated treatments [17-23]. Zeolites have been useful, mainly as ion exchangers, molecular sieves, adsorbents, and catalysts. The resultant zeolitic materials have great potential to be developed as high-efficiency low-cost adsorbents with applications in environmental problems [24-27]. In this paper, several fusion, aging, and hydrothermal conditions in the synthesis of zeolites were tested, promoting the formation of different types of zeolites and hopefully, more crystalline phases. For that reason, an investigation into the synthesis of zeolites from natural clinker on a laboratory scale via two fusion methods and two aging methods is carried out. Several parameters were selected: the alkaline activator/coal by-product ratio for a fusion of 1.2; fusion temperature and time, 800 ºC and 2 h respectively; and the H2O/alkali fused coal by-product ratio = 5 mL/g. The variables were: state of the pre-fusion alkali (solid or liquid); aging method (ultrasonic or static); and aging and reaction times (6,12,24). This study sets the conditions for future synthesis of better and more abundant zeolitic materials from a low-value material.
2. Experimental procedure
2.1. Materials
The starting material used for the synthesis of zeolites was supplied as follows: Natural clinker associated with the spontaneous combustion of coal seams from a late Paleocene formation, which was collected from an open field of Punto Tajo located in Colombia at a latitude of 10°59'09.9'' N and a longitude of 72°43'07.59'' W and an altitude (MSL) of 108.691m. It was prepared prior to synthesis, using the following steps: rough crushing with a Retsch Jaw Crusher BB 200 to ~ 2mm, milling with a Retsch RM100 mortar grinder mill to clay particle size, and sieving with a 200 mesh Ro-Tap sieve shaker; 63 mm particles selected for zeolite synthesis. The reagents used to activate this coal by-product was sodium hydroxide, NaOH, as pellets (97%, from Carlo Erba Reagents), and distilled water using standard purification methods.
2.2. Zeolite synthesis
The synthesis of zeolites developed using two types of alkaline fusions prior to two aging methods and the hydrothermal treatment. Optimal conditions for the synthesis of zeolites from coal by-products with a maximum value of cation exchange capacity are a coal by-product/alkaline activator ratio of 1:1.2 [17-18]. Therefore, alkali hydroxide powder was mixed with natural clinker using two methods, one with dry alkali and the other with alkali dissolved in water forming a saturated solution. The resultant mixture calcined at 800 °C for 2 h. The alkaline reagent added to the starting material acts as an activator agent during fusion. The alkali-fused products were then dissolved in water (H2O/alkali fused starting material ratio = 5 mL/g), under stirring conditions for 30 minutes with a speed of 600 RPM in a VELP Scientifica magnetic stirrer until the reaction gels were homogenized and were then aged for 6, 12 and 24 hours under static and ultrasonic conditions. The mixtures transferred into 65 ml polytetrafluoroethylene (PTFE) bottles and then crystallized under static conditions at 80 °C for 6, 12 and 24 h. After that, the obtained zeolite was filtered and washed with distilled water. Finally, the product was dried at 60°C for two hours.
2.3. Characterization
The X-ray diffraction patterns of the raw materials and synthesized products were recorded in a BRUKER D8 ADVANCE diffractometer operating in Da Vinci geometry equipped with an X-ray tube (Cu-Ka1 radiation: l = 1.5406 Å, 40 kV and 30 mA) using a nickel filter and a 1-dimensional LynxEye detector. A fixed antiscatter slit of 8 mm, receiving slit (RS) of 1 mm, soller slits of (SS) of 2.5º and a detector slit of 0.6 mm were used. Data collection was carried out in the 2q range of 3.5-70°, with a step size of 0.01526° (2q) and had a counting time of 0.4 s/step. Phase identification performed using the crystallographic database Powder Diffraction File (PDF-2) from the International Centre for Diffraction Data (ICDD). The morphology of the raw materials and synthesized zeolites was examined by scanning electron microscopy (FEG Quanta 650), under the following analytical conditions: magnification = 1000-20000x, WD = 7.0-11.0 mm, HV = 10 kV, signal = SE and Z CONT, detector = BSED and ETD. The existence of a zeotype framework was confirmed by Fourier transform infrared spectroscopy in the 4000-400 cm-1 region by using a BRUKER TENSOR 27 spectrometer at 20°C and applying 30 scans per sample. However, only the 1250-400 cm-1 region was discussed, because this is where the spectra showed remarkable changes. A BRUKER ATR PLATINUM cell used.
3. Results and discussion
3.1. Chemical and mineralogical analyses of natural clinker
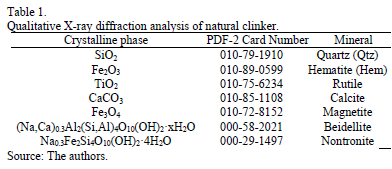
According to Ríos et al. [2] natural clinker presents appropriate SiO2/Al2O3 ratios to be used as starting materials for the synthesis of zeolitic materials with a high cation exchange capacity and high crystallinity. SiO2/Al2O3 ratios are important for predicting the success in the process of zeolite synthesis, although it is also important to consider the occurrence of impurities in the starting material [28]. According to Ríos and co-workers [2,27-29], the chemical composition of the raw natural clinker displays variations of up to 15 wt% among its major oxides (SiO2, Al2O3, K2O, and CaO), which probably reflects local heterogeneity related to small changes in the original sedimentary rock. Natural clinker has very high contents of SiO2 (68.15%) and Al2O3 (19.44%) with a SiO2/Al2O3 ratio = 3.5. Natural clinker shows very low contents of Ca and S, and a high content of Fe associated with the presence of hematite, which is very important taking into account that Fe-bearing minerals, mainly magnetite, can show an inert behavior, and Ca-bearing phases can act as a zeolite synthesis inhibitor through the formation of calcium silicate hydrate phases [9]. A qualitative X-ray diffraction analysis was useful in identifying the crystalline phases of natural clinker using the International Centre for Diffraction Data's (ICDD) PDF-2. Table 1 summarizes this.
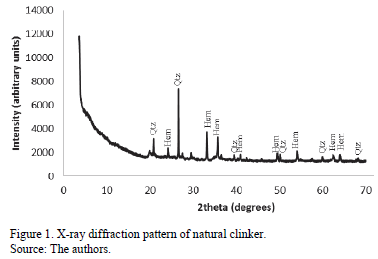
Fig. 1 illustrates the X-ray diffraction for natural clinker. It can be seen that the major crystalline phases found in this geomaterial are quartz and hematite.
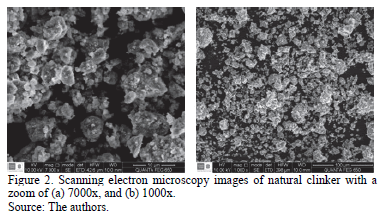
Scanning electron microscopy images of natural clinker (Fig. 2) reveal a roundish agglomerated type surface. Scanning electron microscopy was also used for making a quantitative elemental analysis in back-scattered electrons mode. The EDS spectrum (not shown in this study) of natural clinker reveals that it mainly consists of O, Al, Si, and Fe elements, with minor Mg, Ca, and K. The mass ratios of O:Si:Al:Fe were 30.31:36.56:18.96:6.57.
The morphology of the synthesized zeolites revealed by scanning electron microscopy micrographs was studied in order to choose the zeolites with the most abundant crystalline phases. It was discovered that the morphology was richer and more crystalline phases were obtained when solid clinker-solid NaOH fusion was carried out and the aging method was static.
3.2. Zeolites from fusion of solid clinker and solid sodium hydroxide
The main as-synthesized zeolites obtained by this type of alkaline fusion include: faujasite, sodalite, NaA (LTA) zeolite, and vaterite. The synthesis of these zeolites was investigated by the evaluation of some parameters affecting their formation process, such as the aging method (static or ultrasonic), and the hydrothermal reaction time (6, 12, and 24 hours), and under static conditions during the synthesis process.
3.2.1. Microstructural analysis
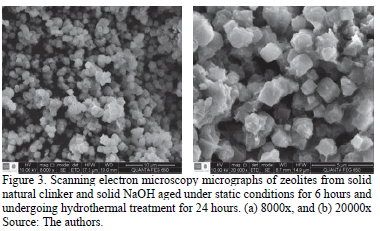
Scanning electron microscopy micrographs showed that six hours of static aging and the longest chosen period of hydrothermal treatment (24 hours) produced more diversity and a higher quality of zeolites. Thus, the best results are in the zeolite produced under 6 hours of static aging and the longest hydrothermal treatment period in this study. Fig. 3 shows how diverse are the morphology and the crystalline phases of this sample.
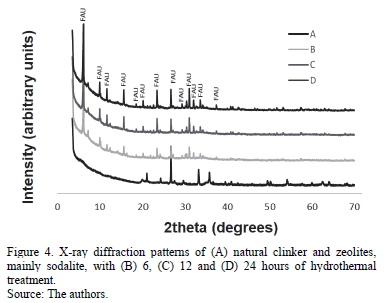
3.2.2. Identification of crystalline phases
Table 2 depicts the results of X-ray diffraction analyses, with the crystalline phases obtained after using solid fusion and static aging.
Fig. 4 presents the X-ray diffraction patterns of the activated natural clinker, which show that faujasite was the major crystalline phase present in the synthesis products. The content of this zeolitic material increased with reaction time, with the highest synthesis yields obtained after 24 hours, with quartz partially dissolved under these experimental conditions. Results show that the conversion of natural clinker was efficient during the synthesis process, and that through the proposed method that was described above, it was possible to transform it into zeolite-like materials, with faujasite as the most abundant phase.
3.2.3. Fourier transformed infrared spectroscopy
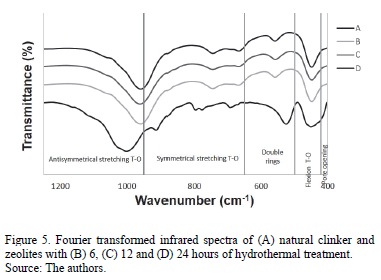
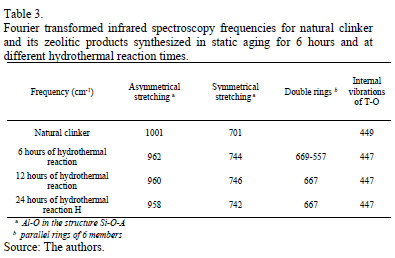
The Fourier transformed infrared spectroscopy spectra of the raw material (natural clinker) and zeolitic materials that were synthesized in static aging for 6 hours and that have different hydrothermal reaction times are illustrated in Fig. 5. The spectrum for natural clinker shows its three wide characteristic bands at 449 cm-1 (T-O bending vibrations), 701 cm-1 (T-O symmetric stretching vibrations) and 1001 cm-1 (T-O asymmetric stretching vibrations). Typical bands of zeolitic materials appear, including T-O bond (T=Al or Si) in the region of 950-1250 cm-1 and the T-O symmetrical stretching in the region of 660-770 cm-1. Bands in the region of 500-650 cm-1are attributed to the presence of double rings (D4R and D6R) in the structure of the zeolitic materials. Moreover, bands in the region of 400-250 cm-1 represent internal vibrations of T-O, while bands in the region of 300-420 cm-1 can be attributed to pore opening. Similar results are reported by Ríos and Williams [18].
These results reveal that, in general, the characteristic frequencies of natural clinker shifted to lower frequencies, although without significantly changing with reaction time.
Table 3 summarizes the characteristic Fourier transformed infrared frequencies obtained for the natural clinker and zeolitic materials.
3.3. Zeolites from fusion of solid clinker and sodium hydroxide in aqueous solution
According to the results obtained by Oviedo et al. [30] the main as-synthesized zeolites obtained by the alkaline fusion followed by the hydrothermal reaction method include: sodalite, faujasite, and zeolite P1. The synthesis of these zeolites was investigated by the evaluation of some parameters affecting their formation process, such as the aging method (static or ultrasonic), and the hydrothermal reaction time (6, 12, and 24 hours).
3.3.1. Microstructural analysis
Scanning electron microscopy micrographs showed that in this case, the zeolites synthesized during 12 hours of static aging and 24 hours of hydrothermal treatment present a poor morphology and four crystalline phases, which is good compared to other synthesized solid clinker-aqueous NaOH fusions. Fig. 6 shows the morphology of this sample.
3.3.2. Identification of crystalline phases
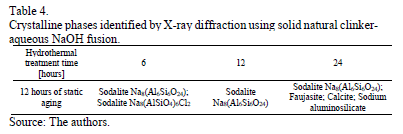
Table 4 depicts the results of X-ray diffraction analyses, with the crystalline phases obtained after using solid natural clinker-aqueous NaOH fusion. Two types of sodalite were obtained in the first 6 hours of hydrothermal treatment; however, only the sodalite Na8(AlSiO4)6Cl24 was obtained after 12 hours. After 24 hours, it crystallized along with faujasite, calcite, and sodium aluminosilicate.
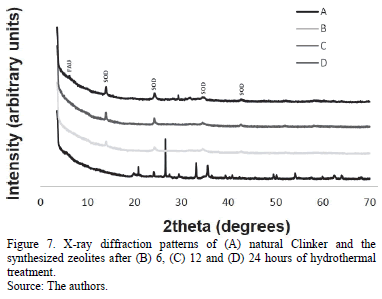
Fig. 7 presents the X-ray diffraction patterns of the activated natural clinker, which show that sodalite was the major crystalline phase present in the synthesis products. The content of this zeolitic material increased with reaction time, with the highest synthesis yields obtained after 24 hours, although it crystallized as a single phase or along with other crystalline phases. Quartz was totally dissolved under these experimental conditions. The conversion of natural clinker was more efficient compared with that observed in the previous method during the synthesis process, and, therefore, the synthesis of zeolite-like materials was successful by the proposed method described above, with sodalite as the most abundant phase.
According to the analysis of X-ray diffraction data, at 24 hours of hydrothermal treatment four crystalline phases were obtained, the most abundant phase was faujasite, which at the same time is the most crystalline one. Sodalite is present in all the synthesized zeolites, and the highest proportion is in the one which had the longest time for hydrothermal treatment.
3.3.3. Fourier transformed infrared spectroscopy
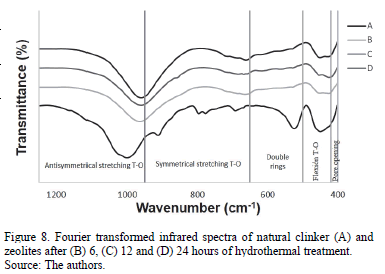
The Fourier transformed infrared spectroscopy spectra of the raw material (natural clinker) and zeolitic materials are illustrated in Fig. 8. The spectrum for natural clinker shows its three wide characteristic bands (at 449, 701 and 1001 cm-1). Characteristic bands of zeolites, including the T-O bond in the region of 950-1250 cm-1 and T-O symmetrical stretching in the region of 660-770 cm-1 were also observed. Bands in the region of 500-650 cm-1 are attributed to the presence of double rings in the zeolite framework. Furthermore, bands in the region of 400-250 cm-1 represent internal vibrations of T-O while bands in the region of 300-420 cm-1and can be attributed to pore opening.
These results reveal that, in general, the characteristic frequencies of natural clinker shifted to lower frequencies, although without significantly changing with reaction time.
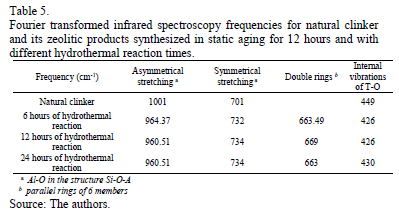
Table 5 depicts the characteristic Fourier transformed infrared frequencies obtained for the natural clinker and zeolitic materials.
A shorter aging time of the hydrogel well-crystallized zeolites formed, whereas aging the hydrogel under longer aging conditions produced poor-crystallized products. In general, results show that sodium hydroxide as-an-activating-agent was efficient in the hydrothermal conversion of natural clinker, similar to what has been reported by Ríos and co-workers [18,27-29]. Recently, Henao and co-workers (personal communication) have been undertaking research on the study of several synthesis parameters on the transformation of natural clinker using aluminum wastes as Al2O3 source, with the production of zeolites Linde Type A and faujasite.
4. Conclusions
Two fusion and aging methods were studied to synthesize zeolites from Colombian natural clinker. The most abundant crystalline phases were obtained from the fusion of solid alkali and solid clinker,, specially the one which took 6 hours of static aging and 24 hours of hydrothermal treatment. This synthesis also showed good results with the alkaline activation of solid clinker and aqueous NaOH obtaining sodalite as a primary phase, with an aging time of 12 hours and a hydrothermal treatment of 24 hours. Aging under ultrasonic conditions in both kinds of fusion methods was counter-productive because the obtained phases were less than the ones that were obtained under static conditions. Therefore, it is concluded that the ultrasonic waves impede the crystallization process. Results from this study reveal the transformation of natural clinker in several synthetic zeotypes with strong similarities compared with previous studies using natural clinker as a starting material in the synthesis of zeolites. A modification of the aging conditions, using an ultrasonic batch, is highlighted here, which can be performed to improve knowledge on the process of nucleation and the crystallization of zeolites. However, further studies should be carried out under well-optimized experimental conditions to successfully prepare highly crystalline zeolites.
Acknowledgments
Authors gratefully acknowledge the Vicerrectoría de Investigación y Extensión of the Universidad Industrial de Santander for financial support through Research Project No. 5454 "Síntesis de zeolitas a partir de residuos sólidos de la industria nacional del carbón para la eliminación de cromo (Cr) de efluentes de industriales". This research forms part of L.F. Ávila and J.A. Lizcano's undergraduate thesis at the Universidad Industrial de Santander. Thanks to the Universidad Industrial de Santander for the use of research facilities (X-ray diffraction, Fourier transformed infrared spectroscopy, and scanning electron microscopy) and the Laboratorio de Consultas Industriales, as well as their human resources. The authors also acknowledge the anonymous referees for their critical and insightful reading of the manuscript and are most grateful to the above-named people and institutions for their support.
References
[1] Höller, H. and Barth-Wirsching, U., Zeolite formation from fly ash. Fortschritte der Mineralogie, 63, pp. 21-43, 1985. [ Links ]
[2] Ríos, C.A., Williams, C.D. and Castellanos, O.M., Synthesis and characterization of zeolites by alkaline activation of kaolinite and industrial by-products (fly ash and natural clinker). Bistua, 4, pp. 60-71, 2006. [ Links ]
[3] Mondragón, F., Rincón, F., Sierra, L., Escobar, J., Ramírez, J. and Fernández, J., New perspectives for coal ash utilization: Synthesis of zeolitic materials. Fuel, 69, pp. 263-266, 1990. DOI: 10.1016/0016-2361(90)90187-U [ Links ]
[4] Kolousek, D., Seidl, V., Prochazkova, E., Obsasnikova, J., Kubelkova, L. and Svetlik, L., Ecological utilization of power-plant fly ashes by their alteration to phillipsite: hydrothermal alteration, application. Acta Universitatis Carolinae Geologica, 37, pp. 167-178, 1993. [ Links ]
[5] Lin, C.-F. and His, H.-C., Resource recovery of waste fly ash: Synthesis of zeolite-like materials. Environmental Science and Technology, 29, pp. 1109-1117, 1995. DOI: 10.1021/es00004a033 [ Links ]
[6] Park, M. and Choi, J., Synthesis of phillipsite from fly ash. Clay Sciences, 9, pp. 219-229, 1995. [ Links ]
[7] Shih, W.-H., Chang, H.-L. and Shen, Z., Conversion of class-F fly ash to zeolites. Materials Research Society Symposium Proceedings, 371, pp. 39-44, 1995. [ Links ]
[8] Amrhein, C., Haghnia, G.H., Kim, T.S., Mosher, P.A., Gagajena, R.C., Amanios, T. and De La Torre, L., Synthesis and properties of zeolites from fly ash. Environmental Science and Technology, 30, pp. 735-742, 1996. DOI: 10.1021/es940482c [ Links ]
[9] Querol, X., Plana, F., Alastuey, A. and Lopez-Soler, A. Synthesis of Na-zeolites from fly ash. Fuel, 76, pp. 793-799, 1997. DOI: 10.1016/S0016-2361(96)00188-3 [ Links ]
[10] Rayalu, S., Meshram, S.U. and Hasan, M.Z., Highly crystalline faujasitic zeolites from fly ash. Journal of Hazardous Materials, 77, pp. 123-131, 2000. DOI: 10.1016/S0304-3894(00)00212-0 [ Links ]
[11] Querol, X., Moreno, N., Umaña, J.C., Alastuey, A., Hernández, E., López-Soler, A. and Plana, F., Synthesis of zeolites from fly ash: An overview. International Journal of Coal Geology, 50, pp. 413-423, 2002. DOI: 10.1016/S0166-5162(02)00124-6 [ Links ]
[12] Murayama, N., Yamamoto, H. and Shibata, J., Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. International Journal of Mineral Processing, 64, pp. 1-17, 2002. DOI: 10.1016/S0301-7516(01)00046-1 [ Links ]
[13] Mouhtaris, Th., Charistos, D., Kantiranis, N., Filippidis, A., Kassoli-Fournaraki, A. and Tsirambidis, A., GIS-type zeolite synthesis from Greek lignite sulphocalcic fly ash promoted by NaOH solutions. Microporous and Mesoporous Materials, 61, pp. 57-67, 2003. DOI: 10.1016/S1387-1811(03)00355-X [ Links ]
[14] Ojha, K., Pradhan, N.C. and Samanta, A.N., Zeolite from fly ash: Synthesis and characterization. Bulletin of Materials Science, 27, pp. 555-564, 2004. DOI: 10.1007/BF02707285 [ Links ]
[15] Inada, M., Eguchi, Y., Enomoto, N. and Hojo, J., Synthesis of zeolite from coal ashes with different silica-alumina composition. Fuel, 84, pp. 299-304. 2005. DOI: 10.1016/j.fuel.2004.08.012 [ Links ]
[16] Ríos, C.A., Williams, C.D. and Roberts, C.L., A comparative study of two methods for the synthesis of fly ash-based sodium and potassium type zeolites with potential use in the purification of wastewaters. Fuel, 88, pp. 1403-1416, 2009. DOI: 10.1016/j.fuel.2009.02.012 [ Links ]
[17] Shigemoto, N., Hayashi, H. and Miyaura, K., Selective formation of Na-X zeolite from fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. Journal of Material Sciences, 28, pp. 4781-4786, 1993. DOI: 10.1007/BF00414272 [ Links ]
[18] Ríos, C.A. and Williams, C.D., Synthesis of zeolitic materials from natural clinker: A new alternative for recycling coal combustion by-products. Fuel, 87, pp. 2482-2492, 2008. DOI: 10.1016/j.fuel.2008.03.014 [ Links ]
[19] Querol, X., Plana, F., Alastuey, A., Lopez-Soler, A., Andres, J.M., Juan, R., Ferrer, P. and Ruiz, C.R., A fast method of recycling fly ash: Microwave assisted zeolite synthesis. Environmental Science and Technology, 31, pp. 2527-2532, 1997. DOI: 10.1021/es960937t [ Links ]
[20] Park, M., Choi, C.-L., Lim, W.-T., Kim, M.-C., Choi, J. and Heo, N.-H., Molten-salt method for the synthesis of zeolitic materials: I. Zeolite formation in alkaline molten-salt system. Microporousand Mesoporous Materials, 37, pp. 81-89, 2000. DOI: 10.1016/S1387-1811(99)00195-X, DOI: 10.1016/S1387-1811(99)00196-1 [ Links ]
[21] Park, M., Choi, C.-L., Lim, W.-T., Kim, M.-C., Choi, J. and Heo, N.-H., Molten-salt method for the synthesis of zeolitic materials: II. Characterization of zeolitic materials. Microporous and Mesoporous Materials, 37, pp. 91-98, 2000. DOI: 10.1016/S1387-1811(99)00195-X, DOI: 10.1016/S1387-1811(99)00196-1 [ Links ]
[22] Hollman, G.G., Steenbruggen, G., and Janssen-Jurkovičová, M., A two-step process for the synthesis of zeolites from coal fly ash. Fuel, 78, pp. 1225-1230, 1999. DOI: 10.1016/S0016-2361(99)00030-7 [ Links ]
[23] Moreno, N., Querol, X., Andrés, J.M., López-Soler, A., Janssen-Jurkovičová, M., Nugteren, H., Towler, M. and Stanton, K., Determining suitability of a fly ash for silica extraction and zeolite synthesis. Journal of Chemical Technology and Biotechnology, 79, pp. 1009-1018, 2004. DOI: 10.1002/jctb.1088 [ Links ]
[24] Moreno, N., Querol, X., Ayora, C., Fernández-Pereira, C. and Janssen-Jurkovičová, M., Utilisation of zeolites synthesized from fly ash for the purification of acid mine waters. Environmental Science and Technology, 35, pp. 3526-3534, 2001. DOI: 10.1021/es0002924 [ Links ]
[25] Moreno, N., Querol, X., Ayora, C., Alastuey, A., Fernández-Pereira, C. and Janssen-Jurkovičová, M., Potential environmental applications of pure zeolitic material synthesized from fly ash. Journal of Environmental Engineering, 127, pp. 994-1002, 2001. DOI: 10.1061/(ASCE)0733-9372(2001)127:11(994) [ Links ]
[26] Querol, X., Moreno, N., Umaña, J.C., Juan, R., Hernández, S., Fernandez-Pereira, C., Ayora, C., Janssen-Jurkovičová, M., García-Martínez, J., Linares-Solano, A. and Cazorla-Amoros, D., Application of zeolitic material synthesized from fly ash to the decontamination of waste water and flue gas. Journal of Chemical Technology and Biotechnology, 77, pp. 292-298, 2002. DOI: 10.1002/jctb.597 [ Links ]
[27] Ríos, C.A., Williams, C.D. and Roberts C.L., Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker and synthetic zeolites. Journal of Hazardous Materials, 156, pp. 23-35, 2008. DOI: 10.1016/j.jhazmat.2007.11.123 [ Links ]
[28] Ríos, C.A., Synthesis of zeolites from geological materials and industrial wastes for potential application in environmental problems. PhD Thesis, University of Wolverhampton, United Kingdom, 2008. [ Links ]
[29] Sandoval, M.V., Henao, J.A., Ríos, C.A, Williams, C.D. and Apperley, D.C., Synthesis and characterization of zeotype ANA framework by hydrothermal reaction of natural clinker. Fuel, 88, pp. 272-281, 2009. DOI: 10.1016/j.fuel.2008.08.017 [ Links ]
[30] Oviedo, J.A., Henao, J.A. and Ríos, C.A., A comparative study on conversion of industrial coal by-products in low SiO2zeolite of faujasite type. DYNA, 79 (176), pp. 105-114, 2012. [ Links ]
J.A. Lizcano-Cabeza, received his BSc. in Chemistry in 2013 from the Universidad Industrial de Santander, Bucaramanga, Colombia. He performed experimental work on the use of coal industry by-products in the synthesis of zeolitic materials using different methods. Currently, he is working as a private consultant.
L.F. Ávila-Ascanio, received his BSc. in Chemistry in 2013 from the Universidad Industrial de Santander, Bucaramanga, Colombia. He performed experimental work on the use of coal industry by-products in the synthesis of zeolitic materials by different methods. Currently, he is working as a private consultant.
C.A. Ríos-Reyes, received his BSc. in Geology in 1989 and his Postgraduate Diploma in University Teaching in 1995 from the Universidad Industrial de Santander, Bucaramanga, Colombia. The Shimane University, Matsue, Japan, conferred his MSc. in Geology in 1999. The University of Wolverhampton, Wolverhampton, England, conferred his PhD in Applied Sciences in 2008. He has been working as a full-time lecturer of the School of Geology at Universidad Industrial de Santander, Bucaramanga, Colombia since 1992, where he has developed his professional experience teaching at a University level over the last 22 years in the field of mineralogy. He has also undertaken much fieldwork in crystalline basement complexes in different areas of Colombia. Over the last six years he has focused his research interests on the use of low-cost raw materials with potential application in the mitigation of environmental problems. Currently, he is the director of the Research Group in Basic and Applied Geology at the School of Geology of the Universidad Industrial de Santander, Bucaramanga, Colombia and the director of the Microscopy Laboratory of the Guatiguará Technological Park.
L.Y. Vargas-Fiallo, received her BSc. in Chemistry in 1980, her Postgraduate Diploma in Environmental Chemistry in 1990, and her MSc. in Analytical Chemistry in 2000, all of them from the Universidad Industrial de Santander, Bucaramanga, Colombia. She has been working as a full-time lecturer in the School of Chemistry at Universidad Industrial de Santander, Bucaramanga, Colombia since 1980, when she received her professional degree. Currently she is the director of the Industrial Consulting Laboratory of the Universidad Industrial de Santander, Bucaramanga, Colombia which bases its work on undertaking monitoring for the characterization of domestic and industrial wastewater analysis, working both in situ and in industry laboratories.