Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.82 no.194 Medellín nov./dez. 2015
https://doi.org/10.15446/dyna.v82n194.46352
DOI: http://dx.doi.org/10.15446/dyna.v82n194.46352
Synthesis of ternary geopolymers based on metakaolin, boiler slag and rice husk ash
Síntesis de geopolímeros ternarios basados en metakaolin, escoria de parrilla y ceniza de cascarilla de arroz
Mónica Alejandra Villaquirán-Caicedo a & Ruby Mejía-de Gutiérrez b
a Composite Materials Group, Faculty of Engineering, CENM, Universidad del Valle, Cali, Colombia, monica.villaquiran@correounivalle.edu.co
b Composite Materials Group, Faculty of Engineering, CENM, Universidad del Valle, Cali, Colombia, ruby.mejia@correounivalle.edu.co
Received: October 18th, 2014. Received in revised form: March 29th, 2015. Accepted: October 22th, 2015.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
Ternary mixtures of geopolymers obtained from the alkaline activation of metakaolin (MK), boiler slag (BS), and rice husk ash (RHA) using a solution of potassium hydroxide were mechanically, thermally, and microstructurally characterized. The geopolymer properties and final microstructures indicate that the addition of BS, despite containing large amounts of unburned material (16.36%), allows for greater densification and greater homogeneity of the geopolymeric gel, which results in greater stability in strength at long curing ages. Substitution of 30% of MK by BS results in an increase in compressive strength of up to 21% and 122% after 28 and 180 days of curing, respectively. These results demonstrate the possibility of the construction sector using geopolymers based on MK and adding BS and RHA to obtain cementitious materials with a lower environmental impact.
Keywords: Geopolymer; metakaolin; boiler slag; rice husk ash.
Resumen
Mezclas ternarias de geopolímeros fueron obtenidas a partir de la activación alcalina de metacaolín (MK), ceniza de parrilla (BS) usando como activador alcalino una mezcla de hidróxido de potasio con ceniza de cascarilla de arroz (RHA). Los materiales producidos fueron caracterizados mecánica, térmica y microestructuralmente. Las propiedades de los geopolímeros y microestructura final indica que la adición de la escoria de parrilla (con grandes cantidades de material sin quemar, 16.36%), permite un mayor grado densificación y una mayor homogeneidad del geopolímero, resultando en una mayor estabilidad de la resistencia para largas edades. La sustitución del 30% del MK por BS genera un incremento de la resistencia a compresión hasta del 21% y 122% después de 28 y 180 días de curado respectivamente. Los resultados aquí obtenidos demuestran la posibilidad de usar geopolímeros basados en MK son adición de BS y RHA en el sector de la construcción para obtener materiales cementicios con bajo impacto ambiental.
Palabras claves: geopolímero, metacaolín, escoria de parrilla, ceniza de cascarilla de arroz.
1. Introduction
Portland cement is the most widely used construction material due to its physicochemical and mechanical properties; however, its production process demands high energy consumption and emits large amounts of CO2, primarily due to the consumption of limestone as raw material and the use of fossil fuels during the clinkering process. Environmental demands in the global industry have led to the development of alternative cementitious materials with lower environmental impacts; among these, alkali-activated cements and geopolymers have emerged. Geopolymers are a diverse group of ceramic materials created by the geosynthesis reaction of an aluminosilicate-type material with an alkaline solution at low temperatures (<100 °C). Thus, the geopolymerization process involves a chemical reaction that results in a network-type tridimensional structure. Geopolymers are known as eco-friendly materials because in addition to the fact that they can use industrial residues and sub-products as raw materials, they require less energy during their synthesis process and acquire great hardness and strength at ambient temperature and at early ages, as early as the first few hours of being produced [1-3]. Geopolymers are non-combustible materials, resistant to high temperatures, and stable against chemical attack [4,5]; in particular, when they are subjected to elevated temperatures, geopolymers undergo a process similar to the sintering of ceramic materials, where the pore sizes are reduced, which densifies the structure and increases the compressive strength. These properties support the potential for widespread implementation of these materials in engineering applications [6,7].
In general, primary-type materials are used as raw materials, i.e., the material is taken directly from natural sources, such as kaolin-type clays or volcanic tuffs. Secondary-type materials are also used, such as waste (ceramic products waste) or industrials sub-products, which also include Fluid Catalytic Cracking [8], fly ash, and slag obtained from different metallurgic processes [9-12]. The function of alkaline activators is to accelerate the dilution of the aluminosilicate source. Alkaline activators include hydroxides, silicates, and carbonates.
Geopolymer synthesis is affected by different parameters, among which, the size and morphology of the precursor particles influence the viscosity of the fresh-state mixture [13]. Thus, geopolymers based on metakaolin (MK) require greater amounts of mixing water due to the laminar morphology and the greater surface area of MK particles [14]; in contrast, the spherical morphology of fly ash (FA) particles means that less mixing water is required, which reduces cracking at elevated temperatures. Other parameters that can affect the synthesis are related to the chemical composition and microstructure of the raw material; thus, it is affirmed that when FA is used, iron and crystalline phases, such as quartz, are unwanted because at temperatures >500°C, cracking by expansion occurs [15,16].
The combination of precursors in geopolymers results in better final properties; in the case of geopolymers based on MK, mixtures of MK and FA [17-21], and MK and blast furnace slag (GBFS) [22] have been investigated. In several cases, reports contradict each other, particularly in the definition of the optimum mixture percentages [12]. Yunsheng [17] studied the durability of MK geopolymeric mortar fibers reinforced with substitutions of up to 50% FA and concluded that with 10% FA, a material with lower porosity, greater impact strength, and a greater tenacity can be obtained; larger percentages adversely affect the properties due to the lower reactivity of FA compared with that of MK. However, in a later study, the same researcher [19] suggested using mixtures of up to 30% FA to obtain acceptable strengths and to immobilize heavy metals. Zhang [20] evaluated percentages of up to 66.7% FA and reported increases in fluidity, setting time, and compressive strength for mixtures with 35.5% FA in MK geopolymers reinforced with polypropylene fibers. In a later study, the same author [18] recommends the substitution of MK for FA in the order of 10%, and attributes the loss of mechanical properties to the increase in porosity of the geopolymeric paste when using higher percentages. Aguilar [21] produced lightweight geopolymeric concretes based on MK enhanced with 25% FA with acceptable properties. It is worth noting that the physicochemical properties of the raw materials in these studies were different. In this sense, the goal in selecting the raw materials for binary and/or ternary mixtures for geopolymeric materials is to seek the best combination of properties, which depends on the application of the final product.
The present study analyzes the mechanical performance of geopolymeric mixtures based on MK and incorporates a sub-product of coal combustion obtained from industrial boilers, which contains a high content of unburned material (boiler slag). It is noteworthy that there is no literature on the use of this material as a partial substitution for MK in the synthesis of geopolymeric materials. In addition, agro-industrial waste is used, i.e., rice husk ash mixed with potassium hydroxide as the alkaline activator. The mechanical compressive strength is evaluated, and techniques, such as thermogravimetric analysis, Fourier transform infrared spectroscopy and scanning electron microscopy, are applied to analyze the microstructure of the developed materials.
2. Experimentation
2.1. Materials
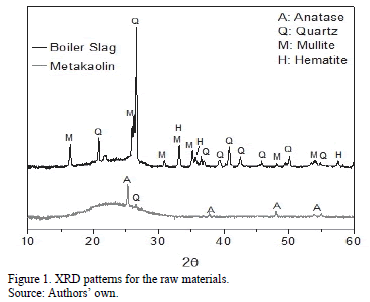
Metakaolin (MK, supplied by the BASF Corporation) and boiler slag (BS, collected from a boiler of PROPAL S.A.) were used as precursor materials. Table 1 shows the chemical composition of the materials determined by X-ray fluorescence (XRF). An X-ray fluorescent spectrometer, MagixPro (PW-2440 Philips) equipped with a Rhodium tube with a maximum power of 4 kW was used. Fig. 1 shows the results of the X-ray diffraction test for the raw materials, where it can be observed that MK is a highly amorphous material; the small crystalline phase signals are attributed to impurities, such as anatase (TiO2, Inorganic Crystal Structure Database, ICSD 154604) and quartz (SiO2,). The BS contained large amounts of quartz, mullite, hematite and in addition, it was possible to identify a slight increase in the base line for the 2q angles between 20°-30°, which suggests the presence of amorphous-state material.
The BS was conditioned by milling in a hammer mill and then in a ball mill for 1 hour until an average particle size of 19.143 µm was obtained. The MK particle size was 7.8 µm.
In both cases, the laser granulometry technique was performed using Mastersizer 2000 equipment. Rice husk ash (RHA) was used as the silica source to prepare the activator. The RHA was obtained via a thermal treatment of the rice husk at 700°C for 2 hours in an electric oven; the amorphous SiO2 content in the RHA was 92%.
2.2. Preparation of the geopolymers
Three geopolymeric systems were studied: E0 (100% MK), E20 (80% MK and 20% BS by weight), and E30 (70% MK and 30% BS). The alkaline solution used as the activating agent was prepared from the RHA mixture with potassium hydroxide pellets in the presence of water. In the E0 mixture, a SiO2/Al2O3 molar ratio of 2.5 and a liquid/solid ratio of 0.4 were used; in the remaining mixtures, the molar ratios were 3.0 and 0.35, respectively; the K2O/SiO2 molar ratio was 0.28 for all mixtures. The specimens were cured at a temperature of 70°C for 20 hours at a relative humidity >90%. Afterwards, they were demolded, wrapped in plastic film to avoid water evaporation, and placed in a chamber at a relative humidity of ~60%; then, the compressive strengths at different curing ages were determined.
2.3. Test
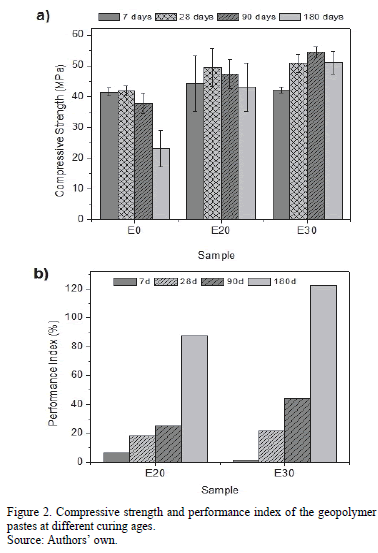
The compressive strength was determined for each of the geopolymeric systems up to an age of 180 curing days using cubic specimens with dimensions of 20 x 20 mm; the test was performed in a universal testing machine, Instron® 3369, at a displacement rate of 1 mm/min. The performance index (P.I) was determined following the eq. (1), where Ex corresponds to E20 and E30
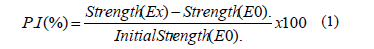
The following instrumental techniques were used for the mineralogical and microstructural analysis of the specimens: X-ray diffraction (XRD) using wide angle diffraction equipment (RINT2000) with a Cu-Ka1 signal at 45 kV and 40 mA with a step of 0.02° in a 2q range of 10°- 60° at a scan speed of 5°/min; Fourier transform infrared spectroscopy analysis (FTIR) was performed with a Perkin Elmer spectrum 100 spectrometer in the transmittance mode in a frequency range of between 450 cm-1 and 4000 cm-1, where the samples were prepared by the KBr compression method; the thermogravimetric analysis (TGA/DTG) of the samples was performed in a nitrogen atmosphere using TA Instruments STD Q-600 equipment at a heating rate of 10 °C/min and up to a temperature of 1250ºC. The collected data were analyzed with Universal Analysis software, version 4.4A from TA Instruments. The scanning electron microscopy (SEM) images were obtained using JEOL JSM-6490LV equipment, which has an INCAPentaFETx3 detector (OXFORD INSTRUMENTS, model 7573).
3. Results and discussion
Fig. 2a shows the results of the compressive strength obtained for the mixtures evaluated up to an age of 180 curing days. Fig. 2b shows that the P.I can be observed when substituting MK by 20 and 30% of BS in the geopolymer; this index represents the strength increment with the added percentage. The average compressive strength at 7 curing days for E20 and E30 was 6.5 and 1.6% better than that of E0, respectively. It can be observed that in general, a decrease in strength occurs at long curing ages (greater than 90 days). This effect is attributed to the curing temperature and time used in the geopolymer synthesis during the initial hardening phase at 70°C for 20 hours [12]. Rovnanik [23] performed a study on the curing temperature (10-80°C) effect on the strength development of the geopolymer based on MK and also reported a decrease in strength as the temperature and time increased, which is associated with a less densified and less compact structure. The author claims that when subjecting the specimen to thermal curing, in particular, at temperatures above 60°C, the polymerization degree increases, which contributes to increased strength during the early ages. However, with rapid setting and hardening, the formation of a lower quality structure with greater porosity is obtained, which is detrimental to the mechanical properties at greater ages [23]. This conclusion coincided with those expressed by other authors [24]. It is noted that the open porosity (ASTM C642) of the geopolymers based only on MK (E0) is 13.7% and 28.9% higher than the open porosity of the E30 and E20 respectively. The addition of BS stabilizes this effect and reduces the porosity, which is seen in the smaller decrease in strength for E20 and E30 at ages of 90 and 180 days. This result is reflected in the performance indexes calculated for E20 and E30 at an age of 180 days, which were 87 and 121%, respectively. Based on compressive strength results of the geopolymer pastes, the samples E0 and E30 were selected. These geopolymer were characterized by FTIR, TGA/DTG and SEM.
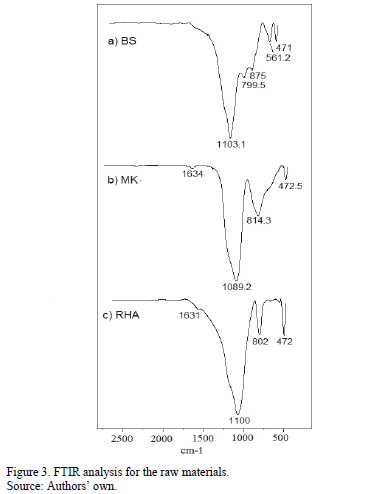
Fig. 3 shows the FTIR analyses of the aluminosilicate precursors (MK and BS) and the silica source used in the preparation of the alkaline activator. The RHA spectrum exhibited a broad band centered at 1100 cm-1, which is associated with the asymmetric stretching vibrational band of the Si-O-Si bonds, and similarly, a vibrational mode at 802 cm-1, which is attributed to the symmetric stretching of Si-O-Si. The MK spectrum contains a broad asymmetric stretching band centered at 1089 cm-1, which is associated with the presence of amorphous structures and with the Si-O-Al/Si bond. A vibrational mode is also observed at 814 cm-1, which is associated with the Al-O bond of AlIV, and at 472 cm-1, which is associated with the Si-O-Si and O-Si-O bond-bending vibrations [25].
The BS spectrum revealed an absorption band between 900-1200°C, which is centered at 1103 cm-1 and associated with the asymmetric stretching vibrations of the Si/Al-O bonds that correspond to quartz, mullite, and minerals found in the XRD spectra of the BS. The signals at 471 cm-1 and 799.5 cm-1 are associated with symmetric stretching vibrations of the Si-O-Si and O-Si-O bonds due to the presence of quartz in the raw material [26]. The signal at 561.2 cm-1 is attributed to the symmetric stretching vibrations of the Al-O-Si-type bonds [27], which is associated with the octahedral aluminum (AlVI) present in the mullite phase, previously identified in the XRD of the material. The signal located at approximately 1630 cm-1 in RHA and MK is associated with the vibration of the O-H bond, which is characteristic of water molecules being weakly adsorbed on the surface by the samples during their preparation.
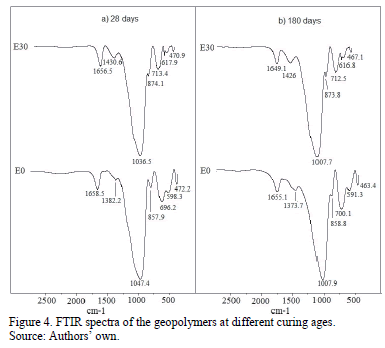
In the FTIR spectra of E0 and E30 (Fig. 4), a broad band of great intensity was identified between 1200 and 800 cm-1, which corresponds to the asymmetric stretching vibration of the Si-O-T bonds (where T corresponds to Si or Al) [14,24]. Generally, this band can be associated with the polymerization degree [29]. Compared with those corresponding to the precursors, this band shifted to lower wavelengths by approximately 50 cm-1 in the geopolymer, as shown in Fig. 4. The E0 signal is at slightly higher wavelengths (1047.4 cm-1) compared with that exhibited by E30 (whose signal is located at 1036 cm-1) after 28 curing days (Fig. 4a). The signals corresponding to the bending vibrations of the Si-O-Si bonds are approximately between 463 and 473 cm-1 in both geopolymers. The signals between 591-616 cm-1 are attributed to the symmetric stretching vibrations of Si-O-Al [30].
The signal identified at ~858 cm-1 corresponds to the asymmetric stretching vibration of the tetrahedrally coordinated Al present in the formed gel, which corroborates the presence of structures composed by tetrahedrons of SiO4 and AlO4 distributed in different configurations, characteristic in these types of materials [14]. The signal at 875 cm-1 identified for geopolymer E30 corresponds to the asymmetric stretching vibration of AlV, associated with mullite from the raw material; this result is an indication that this phase is inactive during the geopolymerization process and remains unaltered. The signals identified at ~697-713 cm-1 correspond to the stretching of the Si-O-Si(Al) bonds, whose intensity indicates the elevated substitution degree of Al in the Si-rich gel, which is characteristic of the formation of amorphous polymers [17,25,31]. This signal is more intense in geopolymer E0.
Likewise, a band located at ~1650-1658 cm-1 is observed, which corresponds to the stretching vibration of the H-OH in molecules of water for reaction products and determines whether they are weakly bound at the surface level or trapped in the structure cavities [32]. The signal at 1430.6 cm-1 is attributed to CO-type bonds associated with stretching vibrations of CO3-2 compounds, in particular, alkaline carbonates generated by the reaction with CO2 in the atmosphere (K2CO3) [33-35] and calcite (CaCO3) generated by the reaction between CO2 in the atmosphere and Ca+2 from BS. Similarly, for the case of E0, the signal is associated with organic matter present in BS.
For both the E0 and E30 samples, the shift of the primary T-O-T band at lower wavelengths (1007 cm-1) in the samples at 180 days can be attributed to a greater substitution of SiO4 tetrahedrons by AlO4 tetrahedrons in the geopolymeric gel, which is a result of a decrease in the SiO2/Al2O3 ratio in the geopolymer matrix with time. The shifts to lower values are due to the lower strength of the Al-O bond, which vibrates at lower frequencies compared with that of the Si-O bond [36,37].
Fig. 5 shows the thermograms for samples E0 and E30 at the age of 180 curing days. The TGA shows that a greater mass loss occurs at temperatures below 400°C, where the mass loss was ~8.70 and 5.99% for E0 and E30, respectively. This loss is attributed to the evaporation of the free water contained in the larger pores (<100 ºC), to the water adsorbed in the gel pores, and to the water bound to the K-A-S-H structure [1]. The decay in the mechanical properties of the sample E0 could be related to the greater content of water weakly bound in this matrix, which was observed in the TGA. In addition, a second weight loss is detected in the DTG for sample E30 in the temperature range of 600-800°C, which is associated to the slag residual coal identified in the XRF test (Table 1), and to the decomposition of the carbonate species previously identified in the FTIR test (Fig. 4), which can be in the form of potassium carbonate or bicarbonate (K2CO3 or KHCO3) [34, 35]. The loss in total weight of up to 1200°C was 9.93% for E0 and 9.44% for E30.
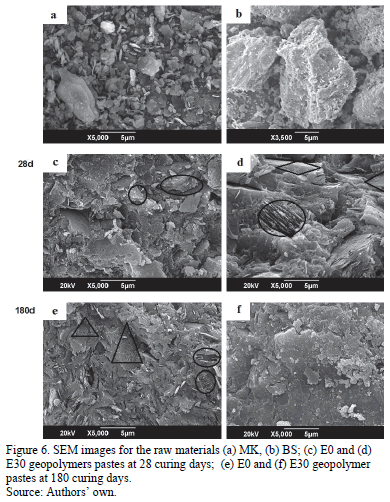
The microstructure of the raw materials and of the geopolymers produced can be observed in the SEM micrographs shown in Fig. 6. Here, a hexagonal laminar morphology is identified for MK in contrast with BS, which contains particles of angular morphology, greater size, and with porous characteristics due to the content of unburned coal (Figs. 6a, 6b, respectively). On the surface of geopolymer E0, at a curing age of 28 days, it is possible to observe small particles of unreacted residual MK (O) embedded in the gel, and in E30, particles of unreacted BS (◊) (Figs 6c, 6d). After 180 days (Fig. 6e), a similar morphology is observed for geopolymer E0, with the presence of pores (D;) and particles of unreacted MK. In contrast, in E30 (Fig. 6f), the surface is more homogenous, soft, and compact, with less porosity and less proportion of unreacted BS or MK particles. The greater porosity in E0 can be attributed to the effect of curing at 70°C, and the greater amount of water in the mixture (liq./sol. ratio of 0.4). Although higher water contents are necessary for workability and mobilization of the alkaline ions during the mixing process, and in the fresh state, at a greater age, this contributes to contraction and cracking and increases porosity in the microstructure of the hardened gel [12,24].
The presence of the BS decreases the water mixing requirements, producing a less porous material, with a more densified and compact structure, which keeps its compressive strength stable for long ages.
4. Conclusions
The geopolymerization of ternary mixtures based on MK, BS, and RHA was successfully performed. The materials produced were mechanically tested and their microstructural characterization was followed up to 180 days of aging. From the results of this investigation, it can be concluded that two industrial sub-products, BS and RHA, can be used along with MK as starting materials in the preparation of ternary geopolymeric mixtures. The high contents of unburned material and crystalline phases, such as quartz and mullite in BS, do not affect the geopolymerization process. The ternary mixture with 30% BS exhibited better mechanical strength for greater ages in comparison with the reference mixture, which indicates that the partial substitution of MK by 30% BS contributes positively to the stabilization of the matrix; strength increments of 121% were obtained after 180 days. In time, the ternary geopolymer showed a greater degree of geopolymerization, a more homogenous surface, and a lower content of unreacted material, which maintains a high strength for long curing times (51 MPa). These materials present properties of interest for their application in the construction sector, in particular, in the production of structural elements, while simultaneously contributing to the reutilization of two industrial residues, BS and RHA, and to a lower environmental impact.
Acknowledgements
The authors would like to thank the Colombian Institute for the Development of Science, Technology, and Innovation (Colciencias) and Universidad del Valle (Centre of Excellence for Novel Materials - CENM) (Cali, Colombia) for their financial support provided for this study.
References
[1] Duxson, P., Lukey, G.C. and van Deventer, J.S.J., Physical evolution of Na-geopolymer derived from metakaolin up to 1000 C. J. Mater. Sci., 42(9), pp. 3044-3054, 2007. DOI: 10.1007/s10853-006-0535-4 [ Links ]
[2] Al-Bakri, M.M., Mohammed, H., Kamarudin, H., Niza, I.K., and Zarina, Y., Review on fly ash-based geopolymer concrete without Portland Cement. J. Eng. Technol. Res., 3(1), pp. 1-4, 2011. [ Links ]
[3] Zhang, Z., Yao, X. and Zhu, H., Potential application of geopolymers as protection coatings for marine concrete I. Basic properties. Appl. Clay Sci., 49(1-2), pp. 1-6, 2010. DOI: 10.1016/j.clay.2010.01.014 [ Links ]
[4] Nicholson, C., Fletcher, R., Miller, N., Stirling, C., Morris, J., Hodges, S., Mackenzie, K. and Schmucker, M., Building innovation through geopolymer technology. Chem. New Zel., 69(3), pp. 10-13, 2005. [ Links ]
[5] Lloyd, R.R., Provis, J.L. and van Deventer, J.S.J., Acid resistance of inorganic polymer binders. I. Corrosion rate. Mater. Struct., 45(1-2), pp. 1-14, 2011. DOI: 10.1617/s11527-011-9744-7 [ Links ]
[6] Duxson, P., Fernández-Jiménez, A., Provis, J.L., Lukey, G.C., Palomo, A. and van Deventer, J.S.J., Geopolymer technology: The current state of the art. J. Mater. Sci., 42(9), pp. 2917-2933, 2006. DOI: 10.1007/s10853-006-0637-z [ Links ]
[7] Komnitsas, K. and Zaharaki, D., Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng., 20(14), pp. 1261-1277, 2007. DOI: 10.1016/j.mineng.2007.07.011 [ Links ]
[8] Martínez-López, C., Mejía-Arcila, J.M., Torres-Agredo, J. y Mejía-de Gutiérrez, R. Evaluación de las características de toxicidad de dos residuos industriales valorizados mediante procesos de geopolimerización. DYNA, 82(190), pp 74-81, 2015. DOI: 10.15446/dyna.v82n189.43136 [ Links ]
[9] Buchwald, A., Weli, M. and Dombrowski, K., Evaluation of primary and secondary materials under technical, ecological and economic aspects for the use as raw materials in geopolymeric binders, Proceedings of the 2nd International Symposium of Non-Traditional Cement and Concrete, 2005. pp. 32-40. ISBN: 80-214-2853-8 [ Links ]
[10] Duxson, P. and Provis, J.L., Designing precursors for geopolymer cements. J. Am. Ceram. Soc., 91(12), pp. 3864-3869, 2008. DOI: 10.1111/j.1551-2916.2008.02787.x [ Links ]
[11] Kovalchuk, G., Fernández-Jiménez, A. and Palomo, A., Alkali-activated fly ash. Relationship between mechanical strength gains and initial ash chemistry. Mater. Constr., 58(291), pp. 35-52, 2008. eISSN: 1988-3226 [ Links ]
[12] Rashad, A.M., Alkali-activated metakaolin: A short guide for civil Engineer - An overview. Constr. Build. Mater., 41, pp. 751-765, 2013. DOI: 10.1016/j.conbuildmat.2012.12.030 [ Links ]
[13] Steveson, M. and Sagoe-Crentsil, K., Relationships between composition, structure and strength of inorganic polymers. J. Mater. Sci., 40(16), pp. 4247-4259, 2005. [ Links ]
[14] Granizo, M.L., Blanco-Varela, M.T. and Martínez-Ramírez, S., Alkali activation of metakaolins: Parameters affecting mechanical, structural and microstructural properties. J. Mater. Sci., 42(9), pp. 2934-2943, 2007. DOI: 10.1007/s10853-006-0565-y [ Links ]
[15] Rickard, W.D.A., Williams, R., Temuujin, J. and van Riessen, A. Assessing the suitability of three Australian fly ashes as an aluminosilicate source for geopolymers in high temperature applications. Mater. Sci. Eng. A, 528(9), pp. 3390-3397, 2011. DOI: 10.1016/j.msea.2011.01.005 [ Links ]
[16] Rickard, W.D.A., van Riessen, A. and Walls, P., Thermal character of geopolymers synthesized from class F fly ash containing high concentrations of iron and a-quartz. Int. J. Appl. Ceram. Technol., 7(1), pp. 81-88, 2010. DOI: 10.1111/j.1744-7402.2008.02328.x [ Links ]
[17] Yunsheng, Z., Wei, S. and Zongjin, L., Impact behavior and microstructural characteristics of PVA fiber reinforced fly ash-geopolymer boards prepared by extrusion technique. J. Mater. Sci., 41(10), pp. 2787-2794, 2006. DOI: 10.1007/s10853-006-6293-5 [ Links ]
[18] Zhang, Z., Wang, H., Zhu, Y., Reid, A., Provis, J. and Bullena, F., Using fly ash to partially substitute metakaolin in geopolymer synthesis. Appl. Clay Sci., 88-89, pp. 194-201, 2014. DOI: 10.1016/j.clay.2013.12.025 [ Links ]
[19] Yunsheng, Z., Wei, S., Wei, S. and Guowei, S., Synthesis and heavy metal immobilization behaviors of fly ash based gepolymer. J Wuhan Univ Technol- Mater Sci., 24(5), pp. 819-825, 2009. DOI: 1007/s11595-009-5819-5 [ Links ]
[20] Zhang, Z-H,, Yao, X., Zhu, H. J., Hua, S-D. and Chen, Y., Preparation and mechanical properties of polypropylene fiber reinforced calcined kaolin fly ash based geopolymer. J Cent South Univ Technol., 16, pp. 49-52, 2009. DOI: 10.1007/s11771-009-0008-4 [ Links ]
[21] Arellano-Aguilar, R, Burciaga-Diaz, O. and Escalante-Garcia, J.I., Lightweight concretes of activated metakaolin-fly ash binders, with blast furnace slag aggregates. Constr. Build. Mater. 24, pp.1166-1175, 2010. DOI: 10.1016/j.conbuildmat.2009.12.024 [ Links ]
[22] Bernal, S.A, Rodriguez, E.D, Mejía-de Gutierrez, R., Gordillo, M.I. and Provis, J.L., Mechanical and thermal characterisation of geopolymers based on silicate-activated metakaolin/slag blends. J. Mater. Sci. 46, pp. 5477-5486, 2011. DOI: 10.1007/s10853-011-5490-z [ Links ]
[23] Rovnaník, P., Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Construction and Building Materials, 24, pp. 1176-1183, 2010. DOI: 10.1016/j.conbuildmat.2009.12.023 [ Links ]
[24] Arellano-Aguilar, R., Burciaga-Díaz, O., Gorokhovsky A. and Escalante-García, J.I., Geopolymer mortars based on a low grade metakaolin: Effects of the chemical composition, temperature and aggregate: Binder ratio. Constr. Build. Mater., 50, pp. 642-648, 2014. DOI: 10.1016/j.conbuildmat.2013.10.023 [ Links ]
[25] Bernal, S., Rodríguez, E., Mejía-de Gutierrez, R., Provis, J. and Delvasto, S., Activation of metakaolin/slag blends using alkaline solutions based on chemically modified silica fume and rice husk ash. Waste and Biomass Valorization, 3(1), pp. 99-108, 2012. DOI: 10.1007/s12649-011-9093-3 [ Links ]
[26] Lee, W.K.W. and van Deventer, J.S.J., Structural reorganisation of class F fly ash in alkaline silicate solutions. Colloids Surfaces A Physicochem. Eng. Asp., 211(1), pp. 49-66, 2002. DOI: 10.1016/S0927-7757(02)00237-6 [ Links ]
[27] Criado, M., Fernández-Jiménez, A. and Palomo, A., Alkali activation of fly ash: Effect of the SiO2/Na2O ratio: Part 1: FTIR study. Microporous Mesoporous Mater., 106(1-3), pp. 180-191, 2007. DOI: 10.1016/j.micromeso.2007.02.055 [ Links ]
[28] Heah, C.Y., Kamarudin, H., Mustafa-Al Bakri, A.M., Bnhussain, M., Luqman, M., Khairul-Nizar I., Ruzaidi, C.M. and Liew, Y.M., Kaolin-based geopolymers with various NaOH concentrations. Int. J. Miner. Metall. Mater., 20(3), pp. 313-322, 2013. DOI: 10.1007/s12613-013-0729-0 [ Links ]
[29] Chindaprasirt, P., Jaturapitakkul, C., Chalee, W. and Rattanasak, U., Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag., 29(2), pp. 539-543, 2009. DOI: 10.1016/j.wasman.2008.06.023. [ Links ]
[30] Panagiotopoulou, C., Kontori, E., Perraki, T. and Kakali, G., Dissolution of aluminosilicate minerals and by-products in alkaline media. J. Mater. Sci., 42(9), pp. 2967-2973, 2006. DOI: 10.1007/s10853-006-0531-8 [ Links ]
[31] Zuhua, Z., Xiao, Y., Huajun, Z. and Yue, C., Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci., 43(2), pp. 218-223, 2009. DOI: 10.1016/j.clay.2008.09.003 [ Links ]
[32] Elimbi, A., Tchakoute, H.K. and Njopwouo, D., Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr. Build. Mater., 25(6), pp. 2805-2812, 2011. DOI: 10.1016/j.conbuildmat.2010.12.055 [ Links ]
[33] Hounsi, A.D., Lecomte-Nana, G.L., Djétéli, G. and Blanchart, P., Kaolin-based geopolymers: Effect of mechanical activation and curing process. Constr. Build. Mater., 42, pp. 105-113, 2013. DOI: 10.1016/j.conbuildmat.2012.12.069 [ Links ]
[34] Onori, R., Alkaline activation of incinerator bottom ash for use in structural applications, PhD. dissertation, Course in Environmental Engineering, Sapienza University of Rome XIII, Italy, 2011. [ Links ]
[35] Prud'homme, E., Michaud P., Joussein E., Peyratout C., Smith A., Arrii-Clacens S., Clacens J.M. and Rossignol S., Silica fume as porogent agent in geo-materials at low temperature. J. Eur. Ceram. Soc., 30(7), pp. 1641-1648, 2010. DOI: 10.1016/j.jeurceramsoc.2010.01.014 [ Links ]
[36] Duxson, P., Lukey, G.C. and van Deventer, J.S.J., Evolution of gel structure during thermal processing of Na-geopolymer gels. langmuir, 22, pp. 8750-8757, 2006. DOI: 10.1021/la0604026 [ Links ]
[37] Wei, Z.Y.S. and Zongjin, L. Preparation and Microstructure of Na-PSDS Geopolymeric Matrix. Ceramics-Silikáty, 53(2), pp. 88-97, 2009. [ Links ]
M.A Villaquirán-Caicedo, has a BSc. in Materials Engineering from Universidad del Valle, Cali, Colombia and a MSc. in Engineering with emphasis in Materials Engineering from the same institution. She is an active member of the Composite Materials Group, category A1-2014 Colciencias, in which she works as a young researcher and research assistant participating in various projects related to the utilization of industrial solid waste. She has been a teaching assistant in courses on composite materials and materials properties in the materials engineering academic program. She is currently a Doctoral candidate in Engineering, with the support of Colciencias Doctoral Scholarship. ORCID: 0000-0001-5145-0472
R. Mejía-de Gutiérrez, has a BSc. in Chemistry, a MSc. degree in Chemical Sciences all of them from Universidad del Valle, Cali, Colombia, and a PhD in Chemical Science (Materials) from the Universidad Complutense de Madrid, Spain. She has extensive experience in the implementation, development and management of research projects related to construction materials (cement, additions, concrete high performance), production of materials from industrial wastes, alkaline activation processes and geopolymerization, composites, ceramic aggregates, and studies on materials durability and corrosion. She has been the director of the GMC since its foundation (1986) and has directed more than 30 research projects. She was the director of the School of Materials Engineering program of the Universidad del Valle, during 2000 and 2006. She is currently the coordinator of the graduate program in the same academic unit. ORCID: 0000-0002-5404-2738