Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.83 no.195 Medellín Jan./Feb. 2016
DOI: http://dx.doi.org/10.15446/dyna.v83n195.42732
In vitro behavior of the dentin and enamel calcium hydroxyapatite in human premolars subjected to high temperatures
Comportamiento in vitro de la hidroxiapatita de calcio de la dentina y del esmalte en premolares humanos sometidos a altas temperaturas
Sebastián Medina a, Liliana Salazar b, Carlos Mejía c & Freddy Moreno d
a School of Basic Sciences at Universidad del Valle, Cali, Colombia. sebastianmedina.c@gmail.com
b School of Basic Sciences at Universidad del Valle, Cali, Colombia. liliana.salazar@correounivalle.edu.co
c School of Dentistry at Universidad del Valle, Cali, Colombia. camejia@emcali.net
d Faculty of Health of Sciences at Pontificia Universidad Javeriana, Cali, Colombia. fmorenog@javerianacali.edu.co
Received: March 21th, 2014. Received in revised form: May 20th, 2015. Accepted: December 10th, 2015.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract
This paper details a cross-sectional, descriptive observational in vitro study of a pseudo-experimental nature that analyzes Electron Microscopy (SEM) by scanning the physical behavior of enamel and dentin calcium hydroxyapatite. The purpose is to describe the separation of these two mineralized dental tissues at the dentin-enamel junction (DEJ) when the teeth are subjected to high temperatures. This study provides scientific evidence that may broaden the discussion on the use of separation of the dentin-enamel junction as a constant and repetitive reliable marker for forensic use (use in forensic sciences) that can contribute to the dental identification process and documentation in a legal medical autopsy, given a situation in which bodies or human remains have been burned, carbonizated or incinerated.
Keywords: Calcium hydroxyapatite, enamel, dentin, dentin-enamel junction, high temperatures, odontological identification, forensic dentistry.
Resumen
Estudio observacional descriptivo de corte transversal y de naturaleza pseudo-experimental in Vitro que analiza, a través de microscopía electrónica de barrido (MEB), el comportamiento físico de la hidroxiapatita de calcio del esmalte y la dentina; con el propósito de describir la separación de estos dos tejidos mineralizados dentales a nivel de la unión amelo-dentinaria cuando los dientes son sometidos a altas temperaturas. Con ello, se proporciona evidencia científica que amplía la discusión sobre el empleo de la separación de la interfase esmalte-dentina como un marcador fehaciente constante y repetitivo de uso forense, el cual puede contribuir con los procesos de identificación odontológica y documentación de la necropsia médico legal para el caso de cadáveres o restos humanos que resulten quemados, carbonizados o incinerados.
Palabras clave: Hidroxiapatita de calcio, esmalte, dentina, unión amelo-dentinaria, odontología forense, identificación odontológica.
1. Introduction
In Colombia, deaths involving the burning, carbonization and incineration of human body [1,2] have increased throughout the first decade of the twenty-first century. This state hinders identification procedures that are commonly employed methods in forensic sciences [3], including technically and scientifically supported procedures by forensic dentistry such as restorative dental treatments [4], or electronic devices implanted in teeth. These require a knowledge of the biological behavior of interfaces between dental tissues and different biomaterials [5].
Teeth are the best preserved organs in extreme conditions (including temperatures) [6]. However, there is little research on the changes that occur in dental tissues when they are subjected to high temperatures. Early in vitro studies focused mainly on the macroscopic description of the structural changes, but did not develop markers with sufficient macroscopic and microscopic scientific support that would allow the application of the results to procedures that help identify a human corpse or human burned, carbonized or incinerated remains [7].
Several studies [8,10,11] have reported that as temperature increases, enamel is detached from dentin at the dental-enamel junction (DEJ). This has been associated with the burning of organic matrix components (non-fibrillar component -glycoproteins, glycosaminoglycans and proteoglycans- and fibrillar component -collagen-), and the physicochemical changes of the inorganic component (calcium hydroxyapatite crystals) of these mineralized dental tissues. It results in decreased tissue volume and loss of DEJ continuity; therefore, the purpose of this study is to describe in vitro behavior of dentin and enamel calcium hydroxyapatite in human premolars that are subjected to high temperatures and, more specifically, to determine whether there is a causal relationship between high temperatures and the separation of the DEJ phenomenon.
2. Dentin
Dentin is a mineralized biological tissue that is composed of 70% inorganic material (hydroxyapatite crystals and trace elements), 18% organic material (type I collagen fiber and proteins such as osteonectin, osteopontin, Osteoclastin-like dentin Gla protein, dentin phosphorine, dentin matrix protein and dentin sialoprotein), and 12% water. Moreover, this tissue has dentinal tubules mainly occupied by odontoblastic processes, and cell specializations extending from pulp odontoblasts, which is why the dentin-pulp complex is described as an integrated structural unit formed by the body of the odontoblast (pulp) and the odontoblast process (dentin) [12-14].
3. Enamel
Enamel is a highly mineralized acellular biological tissue lining like a cap, which is the outer surface of mammals' teeth. Given that this is the hardest and most resistant tissue in the body, the function of enamel is to protect the dentin-pulp complex. It is constituted of 95% inorganic material (calcium hydroxyapatite crystals), 2% organic material (proteins such as amelogenin, enamelin, ameloblastin and tuftelin, among others), and 3% water. As a mineralized tissue, enamel has a functional unit called a "prism", "cane" or "enamel rod", which corresponds to a number of calcium hydroxyapatite crystals parallel to the longitudinal axis of the prism. These are packaged in an organizational pattern, cylindrical in shape and arranged in rows of horizontal alignment. This arrangement occupies the entire thickness of the enamel, except the part located near the dentin, which is not in aprismatic [12-14].
4. Dentin-enamel junction
From when they develop, dentin and enamel are linked by an interface called dentin-enamel junction (DEJ), which is a common starting point for both tissues. This guarantees that dentin and enamel calcium hydroxyapatite crystals never come into contact, as the junction is constituted by amorphous regions the extracellular matrix of which were not completely mineralized: these are respectively called the mantle dentin and aprismatic enamel [15,16].
As described above, the progressive and opposite mineralization of both DEJ tissues begins with the deposition of calcium and phosphate ions on the extracellular matrix. Thus, the DEJ's solution of continuity is due to the hydroxyapatite crystal growth, which results from the synthesis of the organic extracellular matrix and its subsequent mineralization in both tissues, first of the dentin and the enamel immediately after. Therefore, the DEJ corresponds to a transition zone with a scalloped or corrugated appearance between the enamel and the dentin [17,18]. The scallop pattern is represented on the surface of the dentine by a series of peaks that rise towards the enamel, creating a series of concave tunnels with a "honeycomb" aspect that correspond to convexities on the surface of the enamel that belong to groups of enamel prisms [18,19]. The DEJ is therefore a complex mechanism of attachment, the primary function of which involves the formation of an activity surface for the odontoblasts and the ameloblasts to secrete dentin and enamel respectively. Once the tooth is completely formed it contributes to the biomechanical integrity of the structure of which these two tissues are composed [20-22].
5. Calcium hydroxyapatite
Robinson et al [23], expressed in their study undertaken with atomic force microscopy that the development of the ultrastructure of mineralized dental tissue encompasses a series of spherical subunits with a diameter that is similar to a hydroxyapatite crystal (300 and 500 nm). As such, each nanosphere forms an enucleation center that is made of amorphous calcium phosphate and stabilized by the protein components of the extracellular matrix. The longitudinal fusion of nanospheres constitutes a typical hydroxyapatite crystal.
Calcium hydroxyapatite crystal (CHC) corresponds to a biomineral formed by crystalline calcium phosphate that is stored in mineralized tissues, and represents 99% of calcium deposit and 80% of total phosphorus in the body. These calcium phosphates are spherical beads of octacalcium phosphate, arranged in three-dimensional calcium hydroxyapatite crystals, which eventually form enamel prisms [12-14]. Thus, octacalcium phosphate has been recognized as the precursor of hydroxyapatite [24] as it contributes to the formation of the initial mineral phase of the extracellular matrix during biomineralization, and to the subsequent formation of calcium hydroxyapatite [2] by crystallization.
6. Dental tissue mineralization
Biomineralization is the process by which specialized mineralized connective tissues (bone, cartilage, enamel, dentin and cementum) are built by the deposition of a mineral or inorganic phase on the organic extracellular matrix. Regarding the mineralized tissues in teeth, the biomineralization occurs by deposition of calcium hydroxyapatite crystals on the extracellular matrix during the process of amelogenesis (enamel), dentinogenesis (dentin), cementogenesis (cement) and osteogenesis (alveolar bone) mediated by ameloblasts, odontoblasts, and cementoblast and osteoblasts respectively. It is genetically regulated by a group of extracellular matrix mineralization proteins recognized as SCPP (Secretory Calcium-binding Phosphoprotein) [26-28].
During amelogenesis, which is mineralization of the extracellular matrix of enamel, there is a six-step biological crystal formation process from substitutes: 1. Delimitation (ameloblasts are stimulated and begin the secretion of the extracellular matrix from their Tomes's processes); 2. Existence of a preformed organic matrix (built as a structural frame of proteins secreted by the ameloblasts that put themselves together as nanospheres of amelogenin); 3. Oversaturation of the extracellular matrix (creation of a saturated solution of calcium and phosphate ions secreted by ameloblasts); 4. Control of the crystal cores formation (enucleation or crystal self-assembly to set crystal cores controlled by enamelin, tuftelin, amelogenins, ameloblastins and dentin sialophosphoprotein); 5. Control of growth, morphology and orientation of crystals by the extracellular matrix; and 6. Control of the completion of crystal growth (ripening of CHC and proteolytic degradation of excess organic content in the extracellular matrix). This sequential, longitudinal and progressive growth process, starts at the DEJ and ends at the enamel surface [29-31].
With Regards to dentinogenesis, the dentin mineralization mechanism differs considerably from enamel mineralization. Odontoblasts initiate the biomineralization process, which consists of seven consecutive steps: 1. Fibrillar extracellular matrix synthesis (collagen frame); 2. Capture and storage of intracellular calcium; 3. Local concentration of calcium and phosphate ions; 4. Formation of matrix vesicles for its calcification; 5. Release of vesicles from the odontoblast to the extracellular matrix (within these vesicles, amorphous calcium phosphate dots merge together to form CHC); 6.Vesicles rupture and CHC release; and 7. Orientation of CHC in relation to the collagen fibers [29, 32].
7. Materials and methods
This is a cross-sectional (descriptive observational) in vitro study of a pseudo-experimental nature in which the exposure of the sample was undertaken by convenience. Through scanning electron microscopy (SEM) this paper describes the behavior of calcium hydroxyapatite in dentin and enamel human premolars in order to determine whether there is a causal relationship in the detachment phenomenon that occurs between the dentin and enamel at the DEJ when teeth are subjected to high temperatures.
7.1. Sample collection
Upon obtaining endorsement from the Human Ethics Committee of the Health Faculty at the Universidad del Valle, we proceeded to collect a sample of 60 premolar teeth, healthy under clinical observation (no cavities or fractures).
They had no dental treatment and were obtained from patients who attended the Oral Surgery Clinic in the Dental School at the Universidad del Valle, who required premolar extraction for orthodontic reasons and had previously signed an informed consent.
7.2. Handling and preservation of the sample
Once the teeth were extracted, we proceeded to wash them profusely with tap water to eliminate traces of blood and tissue, and placed them in a dark, tightly sealed container with a Chloramine T fixative solution at 5%. The teeth remained in Chloramine T for a week and were then placed in saline solution at room temperature according to what is stipulated in ISO/DIS 11405:2003 [35].
7.3. Sample distribution
Teeth were Classified and randomized according to the temperature to which they were subjected. A control group of ten teeth, which were not subjected to high temperatures, was established in order to have a control (Table 1).
7.4. Application of high temperatures
Once the sample was collected the intervention groups of teeth were individually placed in small trays made of investment material (Whip mix Serafina®), designed under the protocol established by the Dental Materials Unit of the University of Pavia [8]. We then proceeded to place them in a muffle furnace (Thomas Benchtop 1256®) in groups of 10, starting with 30°C for each temperature range (200°C, 400°C, 600°C, 800°C and 1000°C). For example, ten teeth in the 200°C group were put in the furnace, each one in its corresponding tray, at a temperature range of 30°C to 200°C. The furnace was allowed to cool back to room temperature before we took the trays with the teeth out. The same procedure was repeated for the groups of teeth subjected to 400°C, 600°C, 800°C and 1000°C temperatures. This protocol is a standardized practice at the School of Dentistry, University of Valle [11].
7.5. Management of the teeth after application of high temperatures
An acrylic self-curing resin base (New Stetic®) was made for the control group and for the intervention group that was subjected to 200ºC and 400ºC. These teeth were mounted on a universal testing machine (Tinius Olsen® H50KS®) in a Physical and Mechanical testing Laboratory at the School of Materials Engineering (EIMAT) in the Universidad del Valle, which has a 50 Kilo-Newton capacity. A constant vertical, compressive force was applied to them at a crosshead speed of 1 millimeter per minute with a rounded, hardened steel tip with a 3 mm diameter located between the buccal and palatal or lingual cusps until the software machine (Tinius Olsen Horizon®) detected a fracture and the crown was fragmented. This process was undertaken with the objective of provoking adhesive failure, which separates enamel from dentin by unevenly distributed tensional forces. For the teeth in the intervention group that were subjected to 600°C, 800°C and 1000°C, the tissues were separated with a scalpel (Brad Parker® No. 15) and a carver (Lecron Medix®), as these work well with the DEJ's structural weakness and natural spontaneous fracture after having been exposed to high temperatures. From this we gained samples of enamel and dentin.
7.6. Observation of samples of enamel and dentin
Fragments of enamel and dentin that corresponded to each other were set on a glass slide with cyanoacrylate, coated with a gold film, and observed and photographed through a SEM using a JEOL® JSM 6490 LV® from the School of Engineering Materials (EIMAT) at the Universidad del Valle Engineering School. They had a voltage acceleration range of 0.3 KV to 30 KV from a 3nm vacuum source of electrons at a high voltage acceleration.
8. Results
When a tooth is exposed to high temperatures, structural changes that depend on the maximum temperature reached may occur. Consequently, it can stay intact (at 200°C), or it can be burned - change color and fissures and cracks may form (at 400°C)-, it may be carbonized -reduced to charcoal by incomplete combustion (at 600°C)-, be incinerated - reduced to ashes (at between 800°C - 1000°C)-, or burst -radicular and coronal outbreak (at 1200°C)-. Based on what has been previously stated the results of this study and the subsequent discussion section will be affirmed by describing the changes mentioned and we will focus on the changes that took place in the DEJ region.
8.1. Macroscopic detachment of enamel and dentin at the DEJ
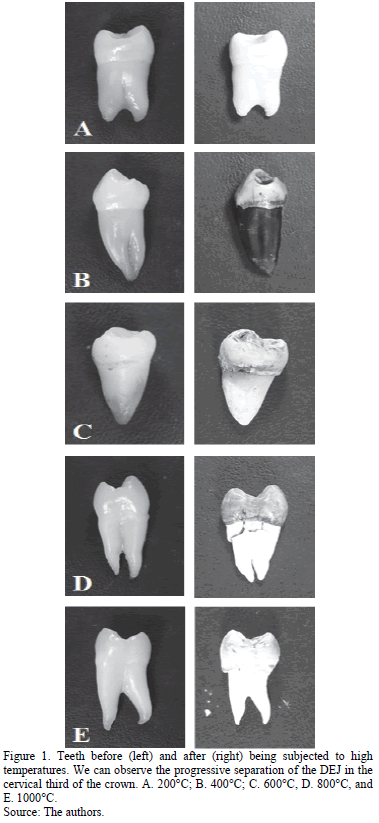
At 200°C there are no macro-structural changes that affect the DEJ beyond the loss of brightness (opacity of the enamel), which is associated with dehydration and cement (Fig. 1A). This was observed when the teeth were compared before and after being subjected to high temperatures. At 400°C, the enamel became opaque and brown (coronal dentin and showed signs of combustion of the organic extracellular matrix); in the cervical region loss of continuity of the enamel was observed by longitudinal and transverse fractures, as well as dentin detachment, indicating loss of DEJ continuity (Fig. 1B). At 600°C, white chalk-colored enamel was found due to incineration and coronal dentin adopted a grayish appearance by a from the carbonization phase to the incineration phase. In the cervical region fragmentation of enamel was observed due to deepening of surface cracks and crevices; this revealed an absolute loss of DEJ continuity (Fig. 1C). At 800°C incinerated enamel adopted a grayish color, and a greater detachment of the enamel dentine and loss of DEJ continuity (Fig. 1D) was observed in the cervical region. Finally, at 1000°C the crown of the teeth turned white because of the burning of enamel and dentin. In the cervical region the DEJ separation was much more evident due to incineration and removal of enamel in addition to the decreased volume of the root with respect to the crown (Fig. 1E).
8.2. CHC of enamel and dentin
SEM was used to observe the microscopic behavior of the inorganic component (mineral phase) of aprismatic enamel and mantle dentin (respective surfaces of the DEJ) represented in the CHC.
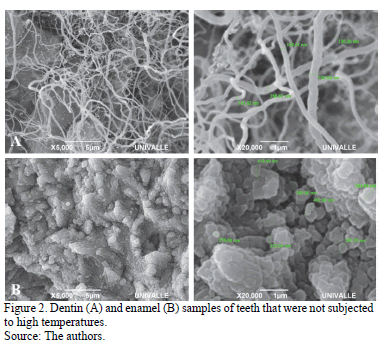
In the teeth samples that were not subjected to any change of temperature there was a regular pattern of calcium hydroxyapatite nanospheres comprising octacalcium phosphate, and a homogenous size distribution within enamel was observed. The presence of the pattern was seen in the collagen fiber network (Fig. 2A and 2B) in the dentine.
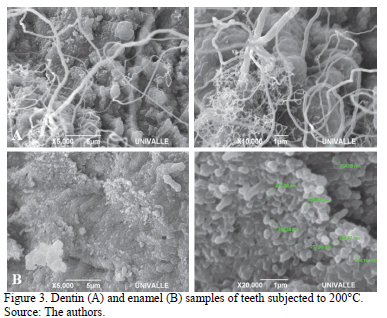
At 200°C, a more compact pattern of calcium hydroxyapatite and dehydration associated with the start of combustion of the low organic component was observed in the enamel. In the dentin, calcium hydroxyapatite began the process of compaction (Fig. 3A and 3B).
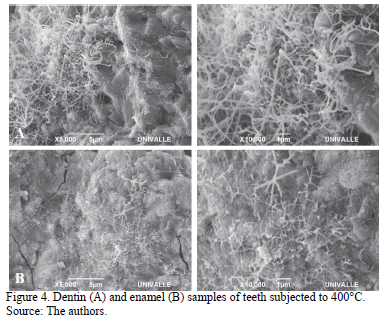
At 400°C the fusion of calcium hydroxyapatite associated with the coalescence of octacalcium phosphate nanospheres that were virtually merged was observed in the enamel. In dentin, compact calcium hydroxyapatite was noticed (Fig. 4A and 4B).
At 600°C, complete melting of the octacalcium phosphate and calcium hydroxyapatite nanospheres was observed in the enamel. In the dentin, a fully fused inorganic component was found and some isolated octacalcium phosphate nanospheres were seen (Fig. 5A and 5B).
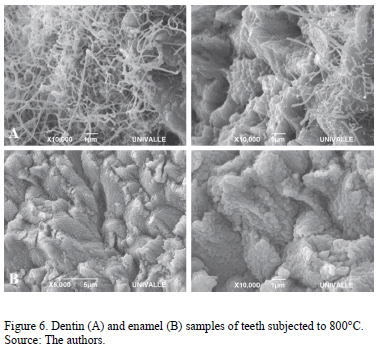
At 800°C the loss of enamel micro-morphology and the enlarged size of the octacalcium phosphate nanospheres were observed. In the dentin a homogeneous melting pattern of hydroxyapatite (Fig. 6A and 6B) was observed.
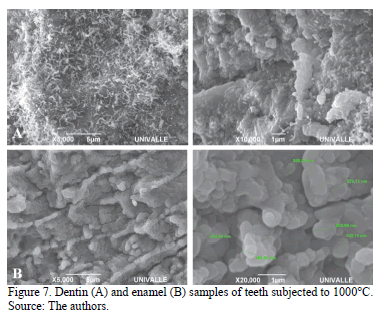
Finally at 1000°C, the integration of octacalcium phosphate nanospheres in separate and larger clusters was noticed in the enamel. Molten dentin continued to have a homogeneous pattern of calcium hydroxyapatite (Fig. 7A and 7B).
9. Discussion
9.1. Dentin and enamel detachment at the DEJ
Merlati et al [7] described DEJ detachment beginning at 400°C. Moreno et al [36], discussed that DEJ detachment begins at 200°C, and that it becomes more evident at 400°C when an outbreak of cervical enamel occurs. At 600°C and at 800°C, full enamel-dentin detachment at the cervical and the middle thirds is observed. When the temperature reaches 1000°C enamel is fragmented and separated from dentin just like the way that a cap is removed. These authors ascribe the detachment phenomenon to the chemical composition of enamel and dentin.
Because enamel has a high inorganic content, which is represented by octacalcium phosphate as CHC, and a low organic content and water (undergoing combustion and evaporation, respectively), the temperature increase alters the organization of such crystals, augmenting their cohesion (thermal contraction). The latter generates the initial appearance of fissures and cracks, which provide a cracked aspect, and ultimately, lead to their fracture.
Also, a higher water and organic content means that dentin takes longer to dehydrate and, because it is protected by enamel itself, in comparison with enamel, it provides a certain spectrum of thermal contraction. This phenomenon is more evident in the cervical third, where the scalloped pattern is reduced.
In the gross examination of the samples that were subjected to temperatures of 200°C, no visible change was observed in the cervical region of the coronal third, as stated by Merlati et al [7] and Moreno et al. [36] At 400°C and at 600°C, enamel and dentin began their process of carbonization. Aprismatic enamel with less inorganic substance quickly lost its low water content and protein, and underwent coalescence of octacalcium phosphate nanospheres, which gradually increased in size and fused together, conforming the CHC. Exactly the opposite happened to the mantle dentin, which has a much higher organic content, which is the reason why that when water was lost and the protein component was denatured, the mantle dentin lost more volume. This caused a burst of enamel by dimensional shrinkage of the dentin at a DEJ level, first at the cervical third and then gradually, up until the middle third. At 800°C and 1000°C, enamel was fully compacted and dentin had gradually begun the process of incineration of the organic component. Thus, the shrinkage phenomenon became much more obvious, which is the reason why the enamel was completely separated from the coronal dentin (in the manner of a cap), as was described by Moreno et al [36].
This spontaneous separation of enamel and dentin was a gradual process that started between 200°C and 400°C and became more evident as the temperature increased up until its total separation that occurred at 1000°C. The explanation of this macroscopic change can be found in the scalloped pattern of the DEJ, which according to Gallagher et al [16], Marshall et al [18], Habelitz et al [20], Imbeni et al [21] and Radlanski and Renzen [22], is very marked in the cusps zone of the DEJ and gradually fades until it disappears in the cervical region. Hence, the latter is the region of least resistance to thermal shock between enamel and dentin, and is the site at which the DEJ initially loses its continuity solution, even at the lowest temperature.
9.2. Behavior of enamel and dentin CHC
Regarding the organization of CHC, no report in the literature has explained in detail their behavior, both in enamel and dentin when subjected to high temperatures.
Holden et al [37] tackled the subject, but studied bone tissue through SEM analysis. They subjected fragments of has disintegrated in such way that the collagen framework is fully exposed. Similarly, the hydroxyapatite crystals are found to be in a spherical shape. Between 800°C and 1000°C incinerated bone looks like white chalk, morphology of concentric lamellae is lost and CHC may present two different morphologies: hexagonal and spherical. Finally from 1000°C to 1600°C, CHCs are fused together; this finding is associated with the sintering process (coalescence at high temperatures) that is much more evident in the inside of the osteon, which is completely disorganized. This is the reason why the classic pattern of the concentric lamellae around the Havers duct cannot not observed.
Subsequently, Holden et al [38], subjected bone fragments to high temperature and analyzed them by X-ray diffraction. They found that the number of CHCs increase as the temperature rises, beginning at 600°C (termed by the authors as re- crystallization). Above 1000°C, CHCs are broken down to calcium oxide, above 1200°C to calcium triphosphate and above 1400°C to phosphate calcium oxide.
Venkatesan and Kim [39] conducted a study in which fish tuna vertebrae were subjected to high temperatures (from 200°C to 1200°C) in order to characterize the behavior of the hydroxyapatite by infra-red spectroscopy, X-ray, TEM and SEM. The authors concluded that as the temperature increases CHCs become agglomerated, beginning at 600°C: temperature at which the micro-crystals grow in size (from 80 to 300nm). In this study, the behavior of bone tissue in terms of color changes determines carbonization signs from 200°C, complete carbonization by burning of the organic matrix (water and protein components) at 600°C and incineration from 800°C. This is similar to what occurs to dentin.
In this study, it was evident that CHC aprismatic enamel and mantle dentin, which constitute the enamel-dentine junction, underwent the same changes that were described in bone tissue. Enamel and dentin start their combustion of extracellular organic matrix at 200°C. At 400°C carbonization starts, which leads to incineration between 600°C and 1000°C. With increasing temperature, the CHC adopts a rounded appearance. This process is most evident in aprismatic enamel given its high inorganic content, whereas in dentin, given its high organic content, changes in calcium hydroxyapatite can only be seen starting from a temperature of 800°C.
10. Conclusions
Microstructural changes (fusion of CHC and sintering of octacalcium phosphate nanospheres) in enamel and dentin, when they are subjected to high temperatures, explain the specific macrostructural changes (DEJ separation) in each temperature range: these being constant and repetitive.
Separation of enamel and dentin at the DEJ can be a reliable medicolegal marker to be able to approximate the temperature to which teeth were subjected. This can be undertaken during the forensic dental identification and documentation of the forensic autopsy process, if there is enough scientific support, in burned, carbonized and incinerated individuals.
It is recommended that the analysis of the behavior of CHC at high temperatures be extended in other mineralized dental tissues (cement, alveolar compact bone and spongy bone), both in interfaces and free surfaces. In order to do this we suggest the design of an animal model in which domestic pig teeth that are articulated on their own socket (not isolated) are subjected to high temperatures for the purpose of being closer to the in vivo conditions.
Acknowledgments
This research was funded by the COLCIENCIAS 2012 Young Researchers internship grant (Programa de Jóvenes Investigadores e Innovadores "Virginia Gutierrez de Pineda" COLCIENCIAS), and by the Vice-President of Research at the Universidad del Valle.
References
[1] Ramírez I.A., Castaño A., González J.O. y Hernández H.W., Homicidios Colombia 2005, en Instituto Nacional de Medicina Legal y Ciencias Forenses. Forensis 2005, datos para la vida. Santa Fe de Bogotá, Imprelibros, 2006. pp. 27-75. [ Links ]
[2] González J.O. y Hernández H.W., Muertes accidentales Colombia 2005. En Instituto Nacional de Medicina Legal y Ciencias Forenses. Forensis 2005, datos para la vida. Santa Fe de Bogotá, Imprelibros, 2006. pp. 254-315. [ Links ]
[3] Pretty, I.A. and Sweet, D., A look at forensic dentistry. Part 1: The role of teeth in the determination of human identity, Br Dental J, 190, pp. 359-366, 1995. DOI: 10.1038/sj.bdj.4800972. [ Links ]
[4] Bowers, C.M. and Bell, G.L., Manual of forensic odontology. 3rd edition. Colorado Springs: American Society of Forensic Odontology, 1997. [ Links ]
[5] Aragón, N., Moreno, F. and Salazar, L., In vitro behavior of interfaces in human molars with an implanted passive RFID microchip and subjected to compression forces, DYNA, 80(178), pp. 5-10, 2013. [ Links ]
[6] Delattre, V.F., Burned beyond recognition: Systematic approach to the dental identification of charred human remains, J Forensic Sci, 45, pp. 589-596, 2000. [ Links ]
[7] Merlati, G., Danesino, P., Savio, C., Fassina, G., Osculati, A., Menghini, P., Observations of dental prostheses and restorations subjected to high temperatures: experimental studies to aid identification processes, J Forensic Odontostomatol , 20(2), pp.17-24, 2002. [ Links ]
[8] Merlati, G., Savio, C., Danesino, P., Fassina, G., Menghini, P. Further Study of restored and unrestored teeth subjected to high temperatures, J Forensic Odontostomatol, 22, pp.17-24, 2004. [ Links ]
[9] Rötzscher, K., Grundmann, C., Benthaus, S., The effects of high temperatures on human teeth and dentures, Int Poster J Dent Oral Med, 6, Poster 213, 2004. [ Links ]
[10] Ferreira, J.L., Espina-de Ferreira, A. and Ortega, A.I., Methods for the analysis of hard dental tissues exposed to high temperatures, Forensic Sci Int, 178, pp. 119-124, 2008. DOI: 10.1016/j.forsciint.2007.12.009. [ Links ]
[11] Moreno, S., Merlati, G., Marín, l., Savio, C. and Moreno, F., Effects of high temperatures on different dental restorative systems: Experimental study to aid identification processes, J Forensic Dent Sci, 1(1), pp. 17-23, 2009. DOI: 10.4103/0974-2948.50883. [ Links ]
[12] Gómez-de Ferrais, M.E. y Campos, A., Histología y embriología bucodental. Segunda edición. Buenos Aires: Panamericana, 2002. [ Links ]
[13] Garant, P.R., Oral cells and tissues. Chicago: Quintessence Books; 2003. [ Links ]
[14] Nanci, A., Ten Cate's Oral Histology: Development, Structure, and Function. 8th ed. St. Louis: Elsevier Mosby, 2008. [ Links ]
[15] Whittaker, D.A., The enamel-dentine junction of human and Macaca irus teeth: A light and electron microscopic study, J Anat, 125(2), pp. 323-335, 1978. [ Links ]
[16] Gallagher, R.R, Demos, S.G., Balooch, M., Marshall, G.W. and Marshall, S.J., Optical spectroscopy and imaging of the dentin-enamel junction in human third molars, J Biomed Mater Res, 64A, pp. 372-377, 2003. DOI: 10.1002/jbm.a.10436. [ Links ]
[17] Chan, Y.L., Ngan, A.H. and King, N.M., Nano-scale structure and mechanical properties of the human dentine-enamel junction, J Mech Behav Biomed Mater, 4(5), pp. 785-795, 2011. DOI: 10.1016/j.jmbbm.2010.09.003. [ Links ]
[18] Marshall, S.J., Balooch, M., Habelitz, S., Balooch, G., Gallagher, G. and Marshall, W., The dentin-enamel junction. A natural multilevel interface, J Eur Ceramic Soc, 23, pp. 2897-2904, 2003. DOI: 10.1016/S0955-2219(03)00301-7 [ Links ]
[19] Smith, T.M., Olejniczak, A.J., Reid, D.J., Ferrell, R.J. and Hublin, J.J., Modern human molar enamel thickness and enamel-dentine junction shape, Arch Oral Biol, 51, pp. 974-995, 2006. DOI: 10.1016/j.archoralbio.2006.04.012. [ Links ]
[20] Habelitz, S., Marshall, S.J, Marshall, G.W.and Balooch, M., The functional width of the dentino-enamel junction determined by AFM-based nanoscratching, J Struct Biol, 135, pp. 294-301, 2011. DOI: 10.1006/jsbi.2001.4409. [ Links ]
[21] Imbeni, V., Kruzic, J.J., Marshal, G.W., Marshal, S.J. and Ritchie, R.O., The dentin-enamel junction and the fracture of human teeth, Nature Mater, 4, pp. 229-232, 2005. DOI: 10.1038/nmat1323. [ Links ]
[22] Radlanski, R.J. and Renz, H., Insular dentin formation pattern in human odontogenesis in relation to the scalloped dentino-enamel junction, Ann Anat, 189, pp. 243-250, 2007. DOI: 10.1016/j.aanat.2006.11.007. [ Links ]
[23] Robinson, C., Fuchs, P. and Weatherell, J.A., The appearance of developing rat incisor enamel using a freeze fracturing technique, J Cryst Growth, 53, pp. 160-165, 1981. DOI: 10.1016/0022-0248(81)90062-2. [ Links ]
[24] Ijima, K.H., Wakamatsu, N., Goto, T., Doi, Y. and Moriwaki, Y., Effects of ca addition on the formation of octa-calcium phosphate and apatite in solution at ph 7.4 and at 37°C, J Cryst Growth, 193(1), pp. 182-188, 1998. DOI: 10.1016/S0022-0248(98)00455-2. [ Links ]
[25] Ijima, M. and Moriwaki, Y., Effects of ionic inflow and organic matrix on crystal growth of octacalcium phosphate; relevant to tooth enamel formation, J Cryst Growth, 198/199, pp. 670-676, 1999. DOI: 10.1016/S0022-0248(98)00986-5. [ Links ]
[26] Veis, A., Mineralization in organic matrix frameworks, Rev Mineral Geochem, 54, pp. 249-289, 2003. [ Links ]
[27] Kawasaki, K., Suzuki, T. and Weiss, K.M., Genetic basis for the evolution of vertebrate mineralized tissue, PNAS, 101(31), pp. 11356-11361, 2004. DOI: 10.1073/pnas.0404279101. [ Links ]
[28] Wilt, F.H., Developmental biology meets materials science: Morphogenesis of biomineralized structures, Dev Biol, 280, pp. 15-25, 2005. DOI: 10.1016/j.ydbio.2005.01.019. [ Links ]
[29] Thesleff, I. and Nieminent, P., Tooth morphogenesis and cell differentiation, Curr Op Cell Biol, 8, pp. 844-850, 1996. DOI: 10.1016/S0955-0674(96)80086-X. [ Links ]
[30] Fincham, A.G, Moradian-Oldak, J. and Simmer, J.P., The structural biology of the developing dental enamel matrix, J Struct Biol, 126(3), pp. 270-299, 1999. DOI: 10.1006/jsbi.1999.4130. [ Links ]
[31] Simmer, J.P. and Fincham, A.G., Molecular mechanisms of dental enamel formation, Crit Rev Oral Biol, 6(2), pp. 84-108, 1995. DOI: 10.1177/10454411950060020701. [ Links ]
[32] Ruch, J.V., Lesot, H. and Begue-Kin, C., Odontoblast differentiation, Int J Dev Biol, 39, pp. 51-68, 1995. [ Links ]
[33] MINISTERIO DE LA PROTECCIÓN SOCIAL., Resolución por la cual se establecen las normas científicas, técnicas y administrativas para la investigación en salud. [Online]. Resolución 008430/1993 de 4 de Octubre [Date of reference: January 2014]. Available at: http://www.minproteccionsocial.gov.co/vbecontent/library/documents/DocNewsNo267711.pdf [ Links ]
[34] ASOCIACIÓN MÉDICA MUNDIAL., Principios éticos para las investigaciones médicas en seres humanos, Declaración de Helsinki. [Online]. Finlandia, junio 1964. [Date of reference: January 2014]. Available at: http://www.wma.net/s/policy/b3.htm [ Links ]
[35] ISO 11405., Dental materials: Testing of adhesion to tooth structure. International Organization for Standardization, 2003. [ Links ]
[36] Moreno, S., León, M.E., Marín, L.y Moreno, F., Comportamiento de los tejidos dentales y de algunos materiales de obturación dental sometidos a altas temperaturas con fines forenses, Colomb Med, 39(1), pp. 28-46, 2008. [ Links ]
[37] Holden, J.L., Phakey, P.P. and Clement, J.G., Scanning electron microscope observations of heat-treated human bone, Forensic Sci Int, 74, pp. 29-45, 1995. DOI: 10.1016/0379-0738(95)01735-2. [ Links ]
[38] Holden, J.L., Clement, J.G. and Phakey, P.P., Age and temperature related changes to the ultrastructure and composition of human bone mineral, J Bone Miner Res, 10(9), pp. 1400-1409, 1995. DOI: 10.1002/jbmr.5650100918. [ Links ]
[39] Venkatesan, J. and Kim, S-K., Effect of temperature on isolation and characterization of hydroxyapatite from tuna (Thunnus obesus) bone, Materials, 3(10), pp. 4761-4772, 2010. DOI: 10.3390/ma3104761. [ Links ]
S. Medina, is a D.D.S. in the School of Dentistry at the Universidad del Valle (Cali, Colombia) and a Young Researcher - COLCIENCIAS 2012. Currently he is a Master's student in the School of Basic Sciences at the Universidad del Valle (Cali, Colombia) and a member of the Soft Tissue and Mineralized Research Group at the Universidad del Valle. His research interests include soft and mineralized biological tissues applied to health sciences. ORCID: http://orcid.org/0000-0002-7000-3499
L. Salazar, has an MSc. in Basic Sciences from the Universidad del Valle, Cali, Colombia. She currently teaches in the School of Basic Sciences at the Universidad del Valle, Cali, Colombia and she is a director of the Soft Tissue and Mineralized Research Group at the Universidad del Valle. She has worked in programs and on projects regarding soft and mineralized biological tissues applied to health sciences. ORCID: 0000-0002-3087-8493
C. Mejía, has an MSc. in Basic Sciences from the Universidad del Valle, Cali, Colombia. He currently teaches in the School of Dentistry at the Universidad del Valle (Cali, Colombia) and is a member of the Soft Tissue and Mineralized Research Group at the Universidad del Valle. His research interests include soft and mineralized biological tissues applied to health sciences. ORCID: 0000-0003-2176-5435
F. Moreno, has an MSc. in biomedical basic sciences from the Universidad del Valle, Cali, Colombia. He currently teaches at the Basic Health Sciences Department in the Faculty of Health Sciences at the Pontificia Universidad Javeriana, Cali, Colombia and the School of Dentistry in the Universidad del Valle, Cali, Colombia. His research interests include soft and mineralized biological tissues applied to forensic sciences. ORCID: 0000-0003-0394-9417