Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.83 no.195 Medellín Jan./Feb. 2016
https://doi.org/10.15446/dyna.v83n195.48130
DOI: http://dx.doi.org/10.15446/dyna.v83n195.48130
Saraz method adjustment for the quantification of ammonia emissions generated in opened or hybrid animal production facilities
Método Saraz ajustado para cuantificar emisiones de amoníaco generadas en instalaciones de producción animal abiertas o híbridas
Fernanda Campos-de Sousa a, Ilda de Fátima Ferreira-Tinôco b, Jairo Alexander Osório-Saraz c, Keller Sullivan Oliveira-Rocha d & Maximiliano Arredondo-Ramirez e
a Department of Agricultural Engineering, Federal University of Viçosa, Viçosa, Brazil. fernanda.sousa@ufv.br
b Department of Agricultural Engineering, Federal University of Viçosa, Viçosa, Brazil. iftinoco@ufv.br
c Department of Agricultural Engineering, Universidad Nacional de Colombia, Medellín, Colombia. aosorio@unal.edu.co
d Department of Agricultural Engineering, Federal University of Viçosa, Viçosa, Brazil. kellersullivan@yahoo.com.br
e Department of Agricultural Engineering, Universidad Nacional de Colombia, Medellín, Colombia. marredondor@unal.edu.co
Received: January 23th, 2015. Received in revised form: October 5th, 2015. Accepted: October 14th, 2015.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract
The objective of this study was to evaluate the efficiency of the Saraz method in order to quantify ammonia emissions generated in opened or hybrid animal production facilities, and to determine an equation for the adjustment method. To do this, we developed beacon equipment, with input and output gas sectors, hoods and absorbent porous material. After the collection, the amount of ammonia captured in the environment was determined in the laboratory. Different ammonia concentrations were evaluated in addition to the different speeds of the exhaust air. Considering the results, it can be concluded that for the situations analyzed the Saraz method is efficient, but as with other methods, with an increase of air velocity and concentration, its efficiency decreases. An equation for the adjustment of the Saraz method was generated to determine the concentration and the rate of ammonia emissions inside animal facilities.
Keywords: air quality; gas emission; natural ventilation.
Resumen
El objetivo de este estudio fue evaluar la eficacia del método Saraz utilizado para cuantificar las emisiones de amoníaco generadas en instalaciones de producción animal abiertas o híbridas y determinar una ecuación ajustada para el método. Para esto, fue desarrollado un equipo con entrada y salida de gases, campanas y materiales porosos absorbentes. Después de la recolección, la cantidad de amoníaco capturado en el medio ambiente fue determinado en el laboratorio. Diferentes concentraciones de amoníaco fueron evaluadas, además de diferentes velocidades del aire. Con base a los resultados, se puede concluir que el método Saraz es eficiente dentro de las situaciones analizadas, pero al igual que otros métodos, con el aumento de la velocidad del aire y la concentración, su eficiencia disminuye. Una ecuación ajustada para el método Saraz fue encontrada, para determinar la concentración y la tasa de emisión de amoniaco en el interior de instalaciones animales.
Palabras clave: calidad del aire; emisión de gases; ventilación natural.
1. Introduction
Ammonia is the most common pollutant found in high concentrations on the premises of animal production facilities [1]. The presence of this gas directly influences the growth of young animals, especially birds. Exposure to an excess of ammonia causes problems and diseases, such as reduced appetite and respiratory rate, respiratory tract lesions, conjunctivitis, and increased susceptibility to viral infections [2]. Besides the economic issues related to ammonia gas in animal husbandry environments, due to it damaging the health of animals and workers, it is also worthwhile to note that ammonia is a greenhouse gas that leads to undesirable environmental consequences when emitted in high concentrations [3]. It may also contaminate water, air and soil, affecting the planet's future of and animal production sustainability [4]. Thus, it is necessary to implement technological strategies to minimize the environmental impact caused by the emission of greenhouse gases [5].
For about two decades, emissions rates and/or concentrations of ammonia in animal production environments have been the focus of studies by researchers in various European countries and in North America, where inventories have been carried out on greenhouse gas emissions, establishing protocols. For these countries, determining ammonia emissions is relatively simple since facilities are closed, and, therefore, there is control over the volume of air in the premises [6].
For regions with tropical and subtropical climates, such as Brazil, determining the concentration of ammonia emissions is much more complex since virtually all livestock shelters are kept open, constituting open or partially open (hybrid) systems, which have interference currents and uncontrollable external wind [7] thermodynamic systems.
Among the methods available to naturally ventilate in predominantly open facilities is that of passive flow. The Saraz method proposed by [8] is noted for its simplicity, efficiency, and applicability to being able to determine ammonia emissions in livestock production in open and general facilities.
The Saraz method was evaluated in field conditions [8] and was considered to be efficient in measuring ammonia emissions from broiler litter coming in very low concentrations, such as 0.5 ppm and 1.0 ppm in ventilation conditions for natural air velocity above 0.1 m s-1. However, according to [8], the method needs to be improved and more research should be conducted into different concentrations and environmental conditions, with the aim of gaining a better understanding of the efficiency and applicability in inventories of greenhouse gases such as ammonia gas.
Despite the great diversity of methods to quantify the concentration of ammonia in the atmosphere , the majority of these are expensive and involve undertaking a series of steps in the laboratory that may contaminate the sample. Therefore, comparison studies are still required, as well as the adaptation and application of methods to quantify ammonia [3].
Thus, there is a need to assess the efficiency of the proposed Saraz method in different environmental conditions, with variations in ambient air velocities, and with different ammonia concentrations studied by [8] in the conditions in the field shelters for livestock. In order to do this, it is important to find a reliable method of calibration, which allows for the actual and possible values of ammonia levels to be quantified. The pickup device employed in the Saraz method is capable of measuring in environments with climate variability and, therefore, it captures the rate of ammonia emissions in a controlled situation by generating an equation for the Saraz method environmental setting.
2. Material and methods
This work was conducted in the Department of Agricultural Engineering at the Federal University of Viçosa in climatic chambers, at Ambiagro - the Center for Research in Agro-industrial ambience, and also at the Engineering Systems and laboratories in the areas of Rural Buildings and Ambience and Energy in Agriculture.
2.1. The beacon equipment development
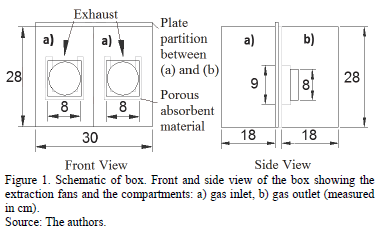
To enable the improvement of the Saraz method, fully sealed beacon equipment was developed, consisting of a box of translucent glass and polycarbonate with the following dimensions: 30.0 x 38.0 x 28.0 cm (length, width, height). This was inserted in the climatic chamber in order to obtain controlled environmental conditions, enabling the analysis in an environment in which the temperature and humidity are controlled and known.
The box, called a normalizing box, was divided into two compartments, an entry sector, and a gas outlet with the same dimensions 30.0 x 18.0 x 28.0 cm (length, width, height). The sector gas inlet was composed of a housing provided with openings for gases to enter(ammonia and fresh air) through silicone tubing connected to the external gas cylinders for the purpose of this study. This compartment was provided with two circular openings of 4.0 cm radius, sealed by absorbent porous material (described in 2.2) positioned above the hoods, which when in use, forced the removal of the outside air to the box. The sector gas outlet, was, in turn, subdivided into two equal sized compartments to allow two samples of air through the porous absorbent material via the exhaust and, hence, two replicates for each variable were analyzed (Figs. 1 and 2).
2.2. Absorbent porous material
The absorbent porous material used was composed of polyurethane sponges with a density D18 (0.0162 g cm-3) and dimensions of 8.0 cm in length, 9.0 cm in height, and 2.0 cm in thickness.
For each repetition, the sponges were impregnated in 25 ml of sulfuric acid (H2SO4), 1.0 mol L-1, and glycerin (C3H5 (OH) 3) 3% v/v before sealing the holes of each of the box's input compartments, according to the method proposed by [9].
2.3. Exhaust fans
The exit velocity of the gas at a known concentration through the porous absorbent material was different for each treatment and had a different speed to the exhaust air (0.1, 1.2, and 2.4 m s-1). This was achieved using hoods installed inside the box that forced the gas through the passage foams, drawing air from inlet compartment to the outlet compartment. The exhaust fans were made by fans or cooler "sleeve bearings" with dimensions of 80 x 80 mm, had a DC voltage of 12 V, a current of 0.15 A, and a speed of 1800 rpm. The speed of the exhaust was controlled by a universal energy source model FT-1462P with a power of 18 W, a maximum load of 1000 mA, a 110/220 Vac input, and seven outputs.
Initially, an exhaust calibration curve was taken to determine the rate of entry of gas into the foam; one hot wire term anemometer (TESTO model 425) was used, measuring with a range from 0 to 20 m s-1 and with an accuracy of ± 0.03 m s-1 and a resolution of 0.01 m s-1.
2.4. Electrochemical Sensors
Electrochemical detectors were used as the instruments to make reference measurements of the various adopted ammonia concentrations (5, 10, 15, 20, and 25 ppm). BW "Gas Alert Extreme Ammonia Detectors", which are compact and affordable, present a variation in measuring accuracy from 0 to 100 ppm 2%, and operate at a relative humidity from 15 to 90% and a temperature of -20 to + 50° C.
The sensors were initially unloaded, and, despite having an automatic setting to grade the security every time you are connected, a simple calibration of all sensors used in the experiment was additionally performed. A total of six sensors were used, three for each sample, with one positioned on each of the three compartments of the normalizing box, the initial compartment for the air containing a known concentration of ammonia and the two sequential compartments with the same dimensions containing the gas after it had passed through the absorbent porous material.
At the end of each collection, a change of the position of sensors occurred, which allowed for a reading of the ammonia level in each sample. Different sensors were positioned in different compartments, thus consecutive readings were avoided. Simultaneously, the sensors were calibrated, and any necessary correction factors were set.
2.5. Climatic chamber
The experiment was conducted in a climatic chamber in order to control the thermal environment for each gas concentration studied. The climatic chamber, located in the Annex to AMBIAGRO, DEA, UFV, was equipped with hot/cold air conditioning of 12,000 BTU h-1, an electric resistance heater with 2000 W of power, a humidifier with a capacity of 4.5 liters and a 300 mL/hr output medium mist. The heater and the humidifier were controlled by an electronic temperature and humidity controller with a 531Ri MT-plus serial communication. Each device was powered on or off automatically in order to ensure temperature control and humidity patterns in the study set.
In this experiment, samples were collected at a temperature of 25° C, and relative humidity was maintained at around 60% in order to simulate creation conditions that were within the range of appropriate comfort for animals such as birds from their second week of life [10]. At this point the generation of ammonia in the production environment is intensified.
2.6. Calibration of the NH3 sensors
The calibration process of the NH3sensors was achieved by using a gas with a known concentration of ammonia and another gas that did not contain ammonia. A gas cylinder with a known ammonia concentration of 25 ppm and an air cylinder with ultra pure synthetic zero with 0 ppm ammonia were used. The cylinders were connected to silicone tubes, and these were connected to measuring instruments following the methodology described by [11].
2.7. Functioning of the normalizing box
At the beginning of each sample collection, the inlet chamber of the housing of the normalizing box received a volume of air with different ammonia concentrations, namely 5, 10, 15, 20, and 25 ppm. These values are situated in the range between the maximum and ideal values for ammonia in an environment for continuous, intermittent and sporadic exposure to animals. These different concentrations of ammonia were obtained from mixtures of ammonia gas up to 25 ppm and with zero ultra pure synthetic air and were monitored by means of electrochemical detectors placed inside the box. As the gases were added to the inlet chamber, the readings were observed by sensors until the desired concentration was obtained. After a time for the sensors to be stabilized and about five equal consecutive readings of the same concentration, we initiated the process of capturing the ammonia absorbed by the porous material that was positioned next to the exhaust fans in contact with the air inlet.
Different speeds of the exhaust air were assessed: 0.1, 1.2, and 2.4 m s-1; these were the upper and lower limits of air typical in some animal production facilities. Birds have a slower speed compared with other animals such as pigs [12-14].
With the exhaust in operation, after the concentration of ammonia was stabilized, a hatch (separating the compartments of input and output gas) was opened that allowed the passage of gas through the porous absorbent material and it to be simultaneously captured. Two other sensors were positioned in the gas exit compartments, one in each compartment, which were monitored to assess possible leaks and to verify the absorption capacity of the absorbent porous material. The final collection time given by the sensors indicated a value of 0 ppm for five consecutive readings, thus indicating that all ammonia present within the gas inlet chamber had been captured by the absorbent porous material.
2.8. Concentration and emission rate of ammonia
The ammonia concentration was determined from the amount of ammonia that the sponges captured, impregnated first with 25 ml of sulfuric acid (H2SO4), 1.0 mol L-1, and glycerin (C3H5 (OH)3) 3% v/v solutions; they were responsible for fixing the diffused ammonia.
After the capture period, the exhaust fans were turned off and the absorbent porous material was removed, stored and refrigerated in plastic wrap to be taken to the laboratory for the ammonia to be extracted according to the Kjeldahl method, in accordance with the methodology adopted by [9].
The ammonia concentration captured by the sponges, that is, the concentration obtained by the Saraz method, was compared to the concentration of ammonia present in the actual normalizing box in terms of the amount of ammonia contained in the foam and calculated in the box, considering the volume of the foam and the normalizing box. Thus, for the amount of ammonia present in the box, the volume of gas input was considered to be equal to 15,120 cm3, taking into account the dimensions of 30.0 cm length, 18.0 cm width, and 28.0 cm in chamber height. For the sponge, the amount was 144 cm3, considering the dimensions of 2.0 cm thickness, 8.0 cm width, and 9.0 cm height. An equation to adjust the concentration obtained from the absorbing porous material due to the expected concentration to be collected by the sponge was generated.
According to the Saraz method, the emissions rate of ammonia through the porous material can be calculated by applying eq. (1):
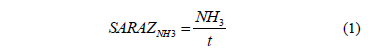
Where:
Saraz NH3: ammonia emission observed in the Saraz method rate (mg s-1);
NH3: Ammonia captured by the absorbent material (mg NH3);
t: Time of exposure to ammonia gas absorbent material (s).
By calculating the quantity values of the NH3 and NH3 concentrations and the rate of ammonia emissions observed with the expected values in the environment, the efficiency was analyzed by the Saraz method according to the time of exposure to the different concentrations of the ammonia situations and different speeds of the exhaust air.
2.9. Experimental design
The experimental design was completely randomized with four replications. The treatments consisted of the combination of five different concentrations of ammonia (5, 10, 15, 20, and 25 ppm) and three different speeds of the exhaust air (0.1, 1.2, and 2.4 m s-1) to analyze the amount of ammonia recovered by the foam and the evaluation of the ammonia emissions rate under different conditions. There were a total of 60 samples (n = 60).
The data amount of ammonia obtained by foam and the ammonia emissions rate were subjected to analysis of variance (ANOVA). When the F test of ANOVA showed difference, the means were compared by Tukey's test. For all statistical analysis, the value of 5% for the probability of type I error was adopted. For an analysis of the model under study, the following hypotheses were tested:
The null hypothesis (H0): concentration data calculated in a real ammonia environment (ERNH3) are equal to the data indirectly through the absorbent material (SARAZNH3).
The alternative hypothesis (H1): concentration data calculated in a real ammonia environment (ERNH3) differ from the data indirectly through the absorbent material (SARAZNH3).
If the alternative hypothesis (H1) was true, and validated using the ANOVA test, we would then carry out a linear regression analysis to determine the coefficients of the model expressed by eq. (2) using the Sigma Plot version 12.0 program.
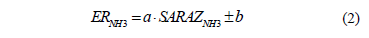
3. Results and discussion
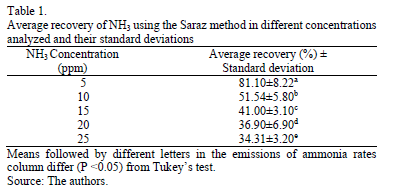
Table 1 presents data for mean recovery of ammonia using the Saraz method for the different concentrations tested. It is observed that, for lower concentrations, the Saraz method displayed the highest ammonia recovery efficiencies of around 81% for the 5 ppm concentration and 51% for the 10 ppm concentration. These results are in agreement with work done by [8], which was undertaken in field conditions with a recovery of volatilized ammonia poultry manure in the 68-82% range.
The Saraz method proves to be more efficient, compared to studies by [15], [16], [17] and [18], which use a collection chamber to determine volatilized ammonia from poultry manure and soil that would normally have maximum values of 70% recovery of ammonia.
This higher recovery efficiency of ammonia through the collector box sponge, compared to what happens in practice, can be explained by the fact that, in this experiment, ammonia present in the ambient air was volatilized. This is unlike what happens in other studies in which the ammonia was still in the process of volatilization in the middle bed or the ground and was then captured by the collector. It was thus dependent on the environment's conditions of volatilization, which can interfere with the recovery efficiency. Similar results were found in [19,17]'s studies, which showed that the efficiency of the collection chamber for open semi-static NH3 varies with the amount of volatilized ammonia, both under greenhouse and in field conditions.
It can be inferred, therefore, that the method used in this study has a good recovery efficiency of ammonia, without an adjustment curve for environments, with concentrations of up to 10 ppm.
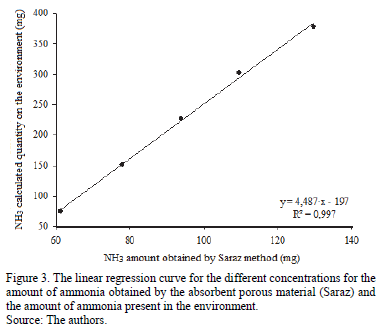
Fig. 3 shows the linear regression curve for the amount of ammonia obtained by the sponge and the amount of ammonia present in different concentrations. It can be observed in Fig. 3 that, independent of the velocity of the exhaust air, one can adjust an equation for any concentration within the values that are being analyzed (up to 25 ppm), since there is a high correlation between the values obtained by foam ammonia and the ammonia values calculated in the environment (R2 = 0.997). This correlation is linear, showing that the method is valid and can be used for the conditions that are analyzed.
Using the regression equation shown in Fig. 3, the calibration equation for the Saraz method, eq. (3), was obtained to determine the actual amount of ammonia captured in situations in which the environment contained less than 25 ppm of ammonia.

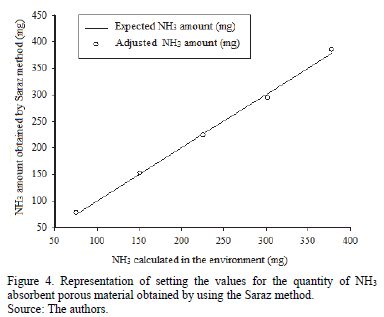
Fig. 4 shows an overlapping set of values with expected values in all observations, which means that an equation fit of the data obtained can be applied. It is therefore indicated that the use of the setting values in the equation for ammonia concentrations in absorbent porous material obtained by the Saraz method proves efficient at any concentration for those tested (up to 25 ppm).
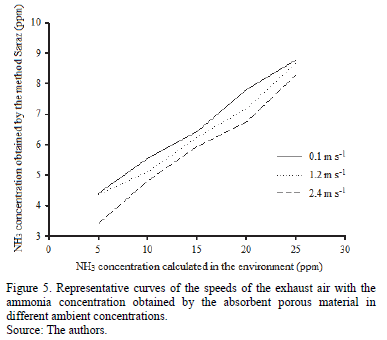
Fig. 5 shows the relationship between the concentration of ammonia present in the environment and the ammonia concentration obtained by the absorbent porous material using the Saraz method, at different speeds of exhaust air. No statistically significant difference (P> 0.05) was found using analysis of variance among the three adopted speeds (0.1, 1.2 and 2.4 m s-1). This indicates that the use of the Saraz method enables the efficient recovery of ammonia without being affected by the air velocity, and, as was expected, the amount of ammonia recovered decreases with increasing air velocity.
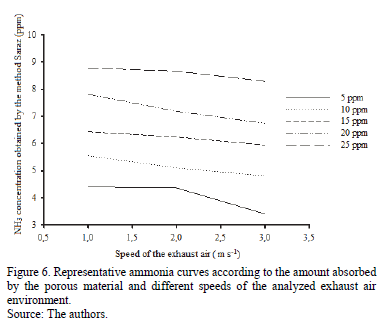
Similarly to Fig. 5, Fig. 6 shows that, independent of the velocity of the exhaust air, the behavior of the curves is the same for all the velocities studied. The amount of ammonia captured by the collector was directly proportional to the concentration of ammonia in the environment, i.e., the higher the amount of water present in the environment, the greater the amount collected by the Saraz ammonia method. In contrast, there is an inversely proportional relation when analyzing the exhaust air speed and the amount of ammonia captured by the collector; the higher the speed of the exhaust air, the smaller the amount of ammonia captured by the Saraz method.
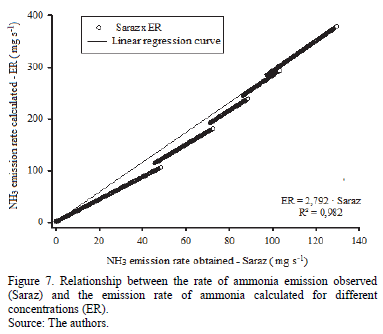
In Fig. 7 shows the regression curve for the rate of ammonia emissions observed from the data obtained by ammonia absorbent porous material (SarazNH3) and the emission rate of ammonia calculated by the expected environmental concentration (ER).
There is high correlation (R2 = 0.982) between the data and the values of the ammonia emission rate obtained by the Saraz method, and the rate values calculated for ammonia emissions in the environment that exhibit a linear relationship, showing that the method is valid under the conditions analyzed.
By using the linear regression curve shown in Fig. 7, it appears that we can estimate the rate of actual emission of ammonia by using a correction factor of 2.79 on the emission rate values obtained by the Saraz method. That is, the real value of the ammonia emission rate (ER) for air velocities between 0 and 2.4 m s-1 and concentrations up to 25 ppm, can be calculated using eq. (4), which is shown below.

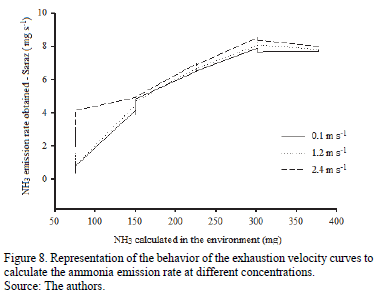
In Fig. 8 (where rates represent ammonia emissions obtained at the various speeds of the exhaust air), the same behavior found in the analysis of the amount of ammonia obtained can be observed. There were no statistically significant differences found (P> 0.05) between the emissions of this gas for each of the different speeds of the exhaust air.
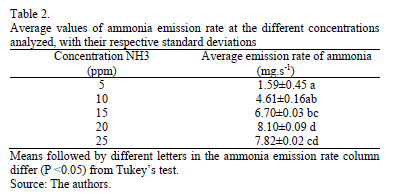
Applying the analysis of variance (ANOVA) to the data rate of ammonia emission, it is possible to observe the significance of this gas' emissions in various concentrations of ammonia in the environment (Table 2). It can be observed that the values of ammonia emission rates increased with increasing concentration in the environment, as was expected.
4. Conclusions
The Saraz method is an efficient method. To determine concentrations of up to 10 ppm it does not need adjustment and presents a good recovery efficiency. For concentrations higher than 10 ppm, the method can be used with confidence through the use of generated equations to adjust both the concentration (eq. 3) and for the rate of ammonia emission obtained by either method (eq. 4), regardless of air velocity.
With air speeds of up to 2.4 ms-1 and ammonia concentrations of up to 25 ppm, the method can be used reliably, but as with other methods, when an increase in air velocity and concentration occurs, efficiency decreases.
Acknowledgments
The authors are thankful to the CNPq, Capes and FAPEMIG.
References
[1] Owada, A.N., Nããs, I.A., Moura, I.J. y Baracho, M.S., Estimativa de bem-estar de frango de corte em função da concentração de amônia e grau de luminosidade no galpão de produção. Eng. Agríc., 27(3), pp. 611-618, 2007. DOI: 10.1590/S0100-69162007000400003 [ Links ]
[2] Osorio, J.A., Tinoco, I.F.F. and Ciro, H.J., Ammonia: A review of concentration and emission models in livestock structures. DYNA, 76(158), pp. 89-99, 2009. [ Links ]
[3] Felix, E.P. and Cardoso, A.A., A method for determination of ammonia in air using oxalic acid-impregnated cellulose filters and fluorimetric detection. J. Braz. Chem. Soc., 23(1), pp. 142-147, 2012. DOI: 10.1590/S0103-50532012000100020 [ Links ]
[4] Carolla, C., Sánches, R. y Montiel, E., Modelo de superfície de respuesta que permite inferior concentración de nitrógeno em "compost" producido a partir de desechos orgánicos. Ing. Investig., 29(3), pp. 128-133, 2009. [ Links ]
[5] Vargas-Nieto, C., Carriazo, J.G. y Castillo, E., Estudio de materiales adsorbents de bajo costo para remover Cr(VI) de efluentes acuosos. Ing. Investig., 31(1), pp. 154-162, 2011. [ Links ]
[6] Osorio-Saraz, J.A., Tinoco, I.F.F., Gates, R.S., Paula, M.O. and Mendes, L.B., Evaluation of different methods for determining ammonia emissions in poultry buildings and their applicability to open facilities. DYNA, 80(178), pp. 51-60, 2013. [ Links ]
[7] Tinoco, I.F.F. y Osorio, J.A., Control ambiental y la agroindustria de produccion animal en el Brasil y America Latina. In: Congreso Nacional de Ingenieria Agricola, Medellín, Colombia, 2008. [ Links ]
[8] Saraz, J.A.O., Tinoco, I.F.F., Gates, R.S., Rocha, K.S.O., Caballero, E.M.C. and Sousa, F.C., Adaptation and validation of a methdology for determing ammonia flux generated by litter in naturally ventilated poultry houses. DYNA, 81(187), pp. 137-143, 2014. DOI: 10.15446/dyna.v81n187.40806 [ Links ]
[9] Osorio, J.A., Determinação experimental e modelagem em CFD das taxas de emissões de amônia de camas de aviários e distribuições de concentrações, temperatura e velocidade do ar no interior de galpões avícolas. Thesis, Department of Agricultural Engineering, Federal University of Viçosa, Viçosa, Minas Gerais, Brasil, 2010. [ Links ]
[10] Cassuce, D.C., Tinôco, I.F.F., Baêta, F.C., Zolnier, S., Cecon, P.R. and Vieira, M.F.A., Thermal comfort temperature update for broiler chickens up to 21 days of age. Eng. Agríc., 33(1), pp. 28-36, 2013. DOI: 10.1590/S0100-69162013000100004 [ Links ]
[11] Amaral, M.F.P., Gates, R.S., Wilkerson, E.G., Overhults, D.G. and Tinôco, I.F.F., Comparison between two systems for ammonia emission monitoring in broiler houses. In: Proceedings, International symposium on air quality and waste management for agriculture, Broomfield, Colorado, 2007. [ Links ]
[12] Medeiros, R., Santos, B.J.M., Freitas, M., Silva, O.A., Alves, F.F.y Ferreira, E., A adição de diferentes produtos químicos e o efeito da umidade na volatilização de amônia em cama de frango. Ciência Rural, 38(8), pp. 2321-2326, 2008. DOI: 10.1590/S0103-84782008000800035 [ Links ]
[13] Vigoderis, R.B., Cordeiro, M.B., Tinôco, I.F.F., Menegali, I., Souza Júnior, J.P. y Holanda, M.C.R., Avaliação do uso de ventilação mínima em galpões avícolas e de sua influência no desempenho de aves de corte no período de inverno. Rev. Bras. Zootec., 39(6), pp. 1381-1386, 2010. DOI: 10.1590/S1516-35982010000600030 [ Links ]
[14] Menegali, I., Tinoco, I.F.F., Carvalho, C.C.S., Souza, C.F. y Martins, J.H., Comportamento de variáveis climáticas em sistemas de ventilação mínima para produção de pintos de corte. Rev. bras. eng. agríc. ambient., 17(1), pp. 106-113, 2013. DOI: 10.1590/S1415-43662013000100015 [ Links ]
[15] Hernandes, R. y Cazetta, J.O., Método simples e acessível para determinar amônia liberada pela cama aviária. Rev. Bras. Zootec., 30(3), pp. 824-829, 2001. DOI: 10.1590/S1516-35982001000300030 [ Links ]
[16] Da Ros, C.O., Aita, C. y Giacomini, S.J., Volatilização de amônia com aplicação de uréia na superfície do solo, no sistema plantio direto. Ciência Rural, 35(4), pp. 799-805, 2005. DOI: 10.1590/S0103-84782005000400008 [ Links ]
[17] Araujo, E.S., Marzola, T., Miyazawa, M., Soares, L.H.B., Urquiaga, S., Boddey, R.M. and Alves, B.J.R., Calibration of a semi-opened static chamber for the quantification of volatilized ammonia from soil. Pesq. agropec. bras., 44(7), pp. 769-776, 2009. DOI: 10.1590/S0100-204X2009000700018 [ Links ]
[18] Alves, A.C., Oliveira, P.P.A., Herling, V.R., Trivelin, P.C.O., Luz, P.H.C., Alves, T.C., Rochetti, R.C. and Barioni-Júnior, W., New methods to quantify NH3 volatilization from fertilized surface soil with urea. Rev. Bras. Ciênc. Solo, 35(1), pp. 133-140, 2011. DOI: 10.1590/S0100-06832011000100012 [ Links ]
[19] Lara-Cabezas, W.A.R. and Trivelin, P.C.O., N-NH3 losses from nitrogen sources applied over unburned sugarcane straw. Rev. Bras. Ciênc. Solo, 14(1), pp. 345-352, 1990. DOI: 10.1590/S0100-06832003000400007 [ Links ]
F. Campos-de Sousa, received her BSc. Eng. in Agricultural and Environmental Engineering in 2012, her MSc. in Agricultural Engineering in the area of Rural Ambience Constructions in 2014, both from the Federal University of Viçosa, Viçosa, Brazil. She is currently a doctoral student in the field of agricultural engineering and rural constructions, a member of AMBIAGRO (Center for Research in Agro-Industrial Ambience and Engineering Systems) at the Department of Agricultural Engineering, Federal University of Viçosa, Brazil. ORCID: 0000-0002-5584-728X
I.deF. Ferreira-Tinôco, received her BSc. Eng. in Agricultural Engineering in 1980, her MSc. in Animal Structures in 1988, her DSc. in Animal Sciencesm in1996, all from the Federal University of Lavras, Minas Gerais state, Brazil. She is currently an associate professor at the Department of Agricultural Engineering of the Federal University of Viçosa, Brazil, and coordinates the UFV branch of the following scientific exchange programs: (1) CAPES/FIPSE University of Illinois and University of Purdue, U.S.A, (2) Umbrella Agreement between UFV and Iowa State University, and the University of Kentucky, (3) Scientific and Technical Agreement between UFV and University of Évora (Portugal), Universidad Nacional de Colombia (Colombia), Iowa State University and University of Kentucky (U.S.A.). She also coordinates the Center for Research in Animal Microenvironment and Agri-Industrial Systems Engineering (AMBIAGRO), and is the President of the DEA-UFV International Relations Committee. ORCID: 0000-0002-8152-0301
J.A. Osório-Saraz, received his BSc. Eng. in Agricultural Engineering in 1998, his Sp in Environmental Legislation in 2001 and his MSc. in Materials Engineering in 2006, all from the Universidad Nacional de Colombia, Medellín, Colombia. In 2011 he received a Dr. in Rural Constructions from the Federal University of Viçosa, Minas Gerais, Brazil. Since 2003 he has been a professor at the Universidad Nacional of Colombia, Medellín, Colombia, teaching and researching in the following areas: design of livestock housing, materials technology for livestock housing, air quality and animal welfare, thermal comfort for animals, use of the CFD tool to predict air motion patterns in agro industry facilities. He is member of AMBIAGRO (Center for Research in Agro-Industrial Ambience and Engineering Systems) at the Department of Agricultural Engineering, Federal University of Viçosa, Brazil and of the research group in agricultural engineering at the Universidad Nacional de Colombia. ORCID: 0000-0003-4867-9158
K.S. Oliveira-Rocha, received he BSc. in Information Systems with an option in Computer Science, in 2005 from the University Center UNA, his MSc. in Agricultural Engineering in 2008, and PhD with an emphasis in Energy in Agriculture in 2012, all from the Department of Agricultural Engineering in the Federal University of Viçosa, Brazil. He has experience in the areas of instrumentation, process control, data acquisition using electronic devices addressable systems, numerical methods applied to engineering, simulation, grain drying and aeration, ventilation systems, machine vision, image processing. He is a member of AMBIAGRO (Center for Research in Agro-Industrial Ambience and Engineering Systems), at the Department of Agricultural Engineering, Federal University of Viçosa, Brazil. ORCID: 0000-0003-4305-4837
M. Arredondo-Ramirez, received his BSc. Eng. in Agricultural Engineering in 2014, at the Universidad Nacional of Colombia, Medellín, Colombia and was then an intern at the UFV. He is currently a MSc. Engineering student in water resources. He is a member of OCEÁNICOS (Research Group about Simulation Software Sea and Engineering Systems from ocean), at the Facultad de Minas, Universidad Nacional de Colombia, Medellin, Colombia. ORCID: 0000-0003-4096-1212