Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
DYNA
versão impressa ISSN 0012-7353
Dyna rev.fac.nac.minas vol.83 no.196 Medellín mar./abr. 2016
https://doi.org/10.15446/dyna.v83n196.50897
DOI: http://dx.doi.org/10.15446/dyna.v83n196.50897
Analysis of working nanofluids for a refrigeration system
Análisis de nanofluidos para un sistema de refrigeración
Diana C. Hernández a, César Nieto-Londoño b & Zulamita Zapata-Benabithe c
a Grupo de Energía y Termodinámica, Universidad Pontificia Bolivariana. Medellín, Colombia. dianacarolina.hernandez@upb.edu.co
b Grupo de Investigación en Ingeniería Aeroespacial, Facultad de Ingeniería Aeronáutica, Universidad Pontificia Bolivariana, Medellín, Colombia. cesar.nieto@upb.edu.co
c Grupo de Energía y Termodinámica, Facultad de Ingeniería Química, Universidad Pontificia Bolivariana, Medellín, Colombia. zulamita.zapata@upb.edu.co
Received: May 28th, de 2015. Received in revised form: November 19th, 2015. Accepted: December 22th, 2015
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract
This paper presents the analysis of nanorefrigerant fluids to improve the thermal efficiency of a refrigeration system. Simulations in ANSYS FLUENT 15.0 were performed with mixtures of refrigerants R113, R123 and R134a, with Al2O3 nanoparticles at 1 vf% and 5 vf% (vf%, fraction volume concentration), flowing through a horizontal tube with a constant wall temperature. A mixture and the k-e turbulent models were employed to obtain results of heat transfer coefficient, temperature and pressure drop for each case. Results show an increment in thermal characteristics by adding 1 vf% and 5 vf% of Al2O3 nanoparticles to the three refrigerants selected. However, the size of the nanoparticle does not affect the thermal properties of nanofluid and the pressure drop does not show a specific pattern of behavior at different concentrations of nanoparticles. Finally, the mixture of R134a with 30 nm of mean diameter size Al2O3 at 1 vf% was selected because of its higher thermal efficiency and its favorable properties as a refrigerant.
Keywords: Nanofluids; Refrigeration system; Thermal efficiency; Heat transfer coefficient.
Resumen
Este trabajo presenta el análisis de nanofluidos para mejorar la eficiencia térmica de un sistema de refrigeración. Se realizaron simulaciones en ANSYS FLUENT 15.0 de la mezcla de refrigerantes R113, R123 y R134a, con nanopartículas de Al2O3 al 1 vf% y 5 vf% de concentración, fluyendo a través de un tubo horizontal con temperatura de pared constante. Se empleó el modelo de mezcla Mixture, y el modelo de turbulencia k-e para obtener resultados del coeficiente de transferencia de calor, temperatura y caída de presión para cada uno de los casos. Los resultados muestran un aumento en las características térmicas de los tres refrigerantes utilizados. Sin embargo el tamaño de las nanopartículas no afecta las características del nanofluido, y no se presenta una tendencia en el comportamiento de la caída de la presión a diferentes concentraciones de nanopartículas. Finalmente se seleccionó la mezcla de R134a y partículas de 30 nm de diámetro de Al2O3 al 1vf% debido a su mayor eficiencia térmica y a sus mejores características como refrigerante.
Palabras clave: nanofluidos; sistema de refrigeración; eficiencia térmica; coeficiente de transferencia de calor.
1. Introduction
1.1. Refrigeration Systems
Refrigeration is a thermal process in which heat transfer is promoted between a system and a refrigerating fluid to remove heat and maintain a low temperature. Refrigeration systems are used for domestic, commercial and industrial applications. Additionally, they are used for food preservation, air conditioners and in the transportation industry. Refrigerants are those kinds of fluids that have the ability to absorb heat at low temperatures and pressures, and yield to higher temperatures and pressures. They can be classified into five groups [1]:
- Halocarbon: CFC-11 or R-11, CFC-12 or R-12, CFC-113 or R-113, CFC-114 or R-114 and CFC-115 or R-115.
- Hydrocarbons (HC): Methane (R-50), ethane (R.170), propane (R-290), n-butane (R-600), and isobutane (R-600a).
- Inorganic compounds: ammonia (R-717), water (H2O), air (R-729), carbon dioxide (R-744) and sulfur dioxide (SO2).
- Azeotropic mixtures: R-502 (48.8% R-22 and R-115 51.2%), R-500 (73.8% R-12 and R-152a 26.2%), R-503 (59.95% R-13 and R-23 40.1%), and R-504 (48.2% R-32 and R-115 51.8%).
- Zeotropic Mixtures: R-401A and R-401B.
Several authors have studied and identified the optimal parameters for optimal performance of the compression refrigeration cycle [1-5]. No fluid is ideal in all aspects; each refrigerant can have negative properties such as toxicity, chemical instability, flammability, high operating pressures or poor thermodynamic properties. Currently, the most widely used fluid in refrigeration and air conditioners is limited to HFCs refrigerants R134a, R32, R125, R143a and mixtures of these, as well as some hydrocarbons (propane and isobutane), ammonia and dioxide carbon [2] .
Historically inorganic natural refrigerants such as R717, R744, R764, R11 or R12 have been used as CFCs and HCFCs such as R22, and R502 mixture [3] . Currently HFCs like R32 and R134a are used as replacement refrigerants, as well as zeotropic as mixtures of HFCs R404A, R407C, R410A, R507 azeotropic mixtures of HFCs and natural hydrocarbons such as R600a and R290. The CFC refrigerants R11 and R12 have been substituted by R123 (HCHC) and R134a (HFC) refrigerants with a reduction in impact on the deterioration of the ozone layer [3]. R134a is still a greenhouse gas, so in the future must be replaced. R152a, CO 2 and R1234yf have been considered as possible replacements of this. However, R152a is a flammable fluid, making it difficult to use and CO2 requires higher working pressure than R134a, which is not practical for a refrigeration system.
Many studies have been undertaken on the impact of refrigerants in the deterioration of the ozone layer and global warming. Efforts have focused on finding an alternative fluid as an ideal substitute. However, the probability of finding an ideal refrigerant is practically zero, due to the number of factors that are involved in the performance of refrigeration systems [6]. R123 has replaced R11 in centrifugal chillers. It is a low pressure, high efficiency refrigerant, and miscible with mineral lubricants. R123 is safer than R11, but a long-term alternative to replace it (R245fa or R245ca) must be found. HCHC are less reactive than CFCs, because of their hydrogen content. R123 has a lifetime in the atmosphere and a lower ODP (Ozone Depletion Potential) than CFCs. However, the Montreal Protocol has limited its use since January 1, 1996 and is expected to gradually decrease its use until 2030 [7] .
HFCs have better environmental characteristics than the CFCs because they contain no chlorine atoms and zero ODP. In [7] the authors present the R134a as an attractive option and a long-term alternative to replace R12 and R22. According to the information reported in the literature, R123 and R134a refrigerants were selected. Zeotropic and azeotropic mixtures were not chosen because their properties and characteristics are more complex for computational analysis to be undertaken. However, these mixtures are not discarded for further analysis. Additionally, testing with refrigerant R113, which has been extensively studied in experimental and computational evidence, was performed in order to have a point of comparison and validation of results.
1.2. Nanofluids
Nanofluids are a suspension of particles between 0 and 100 nm in a base fluid. They have thermophysical properties different to the base fluid due to the addition of metal or metal oxide particles to increase the coefficients of thermal conduction and convection [8,9]. The main characteristic of nanofluids is the ability to enhance heat transfer without altering the base fluid Newtonian behavior with the addition of small concentrations of solid particles [10] .
Experimental and numeric tests have been performed in order to better understand the behavior of these fluids and their characteristics. Studies have focused on thermal conductivity, convective heat transfer coefficient, viscosity, evaporation phenomenon, the influence of particle size and optimal concentration of particles. Some of the advantages to using nanofluids proposed by Choi in [11] are:
- High specific surface area and therefore greater heat transfer surface between particles and fluid.
- High stability of the dispersion where the Brownian motion of particles dominates.
- Reduction of the pumping power in comparison with the base liquid, to achieve an equivalent heat transfer.
- Reduced clogging particles compared to conventional suspensions, promoting miniaturization of the system.
- Adjustable properties by varying the concentration of particles.
In [12] , the authors describe the challenges faced in studying nanofluids and its characteristics such as thermal conductivity, the Brownian motion of particles, migration of these and the variation of thermophysical properties with change in temperature [8] . The long-term stability of the dispersion of nanoparticles is a technical challenge to prevent the accumulation and sedimentation of particles. The pressure drop and higher pumping power should also be considered to determine the efficiency of nanofluids. Other challenges include an increase in viscosity with greater concentration of particles, low specific heat compared to the base fluid, the prediction of thermal conductivity, high costs and production processes.
1.3. Nanorefrigerants
Recently nanoparticles have been used to enhance the thermophysical properties of refrigerants in order to achieve greater efficiency and profitability in refrigeration and air conditioning. In the literature, studies have reported rheological and heat transfer mechanisms to different concentrations of CuO nanoparticles, Al2O3, SiO2, diamond, CNT (carbon nanotube), TiO2 as refrigerants R11, R113, R123, R134a and R141b [12] . The effect of the size of the nanoparticles on heat transfer in mixtures of refrigerant, oil and nanoparticles is investigated experimentally with R113, VG68 oil and Cu particles with diameters of 20, 50 and 80 nm [13] . The results show a maximum increase of 23.8 % in the heat transfer coefficient in a pool nucleate boiling with reduced diameter from 80 to 20 nm.
One of the main factors in the efficiency of a refrigeration system with nanorefrigerants is the heat transfer in the phase change of the heat exchangers (evaporator and condenser). Heat transfer by flow boiling of a nanorefrigerant was studied by Peng et al. [14] using a mixture of R113 and CuO particles. Experimental tests show a 29.7 % increase in the heat transfer coefficient due to the addition of CuO nanoparticles. Peng et al. [14] also proposed a correlation for heat transfer in nanorefrigerants and obtained a deviation of ± 20 % with the experimental results.
Peng et al. [14] propose using the impact factor of the nanoparticle to correct the coefficient of heat transfer of the pure refrigerant. The impact factor of the 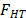 nanoparticle is shown in eq. (1).
nanoparticle is shown in eq. (1).
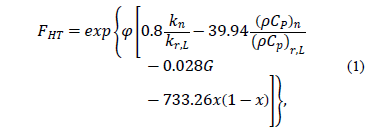
where the subscript "n" and "r, L" represent the properties of nanoparticles and pure refrigerant, respectively. 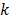 is the thermal conductivity, r is the density,
is the thermal conductivity, r is the density, 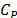 is the isobaric specific heat, j is the volume fraction of the nanoparticles, G is the mass flux and x is vapor quality. To find the heat transfer coefficient, Peng, et al. used eq. (2) [14] .
is the isobaric specific heat, j is the volume fraction of the nanoparticles, G is the mass flux and x is vapor quality. To find the heat transfer coefficient, Peng, et al. used eq. (2) [14] .
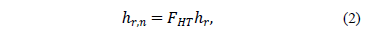
where 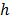 represents the heat transfer coefficient for both, refrigerant and the nanoparticle suspension.
represents the heat transfer coefficient for both, refrigerant and the nanoparticle suspension.
Various combinations of nanoparticles have been studied to analyze the boiling heat transfer by flow boiling of a refrigerant in a horizontal pipe [15] . The nanorefrigerants they used were Cu-Al-R141b, Al 2O3-R141b, and CuO-R141b. They did some experimental tests where the heat transfer coefficient increased by varying the mass fraction of nanoparticles. Cu particles have a better effect on improving the performance of heat transfer of nanorefrigerants due to their high thermal conductivity. Other factors influencing the performance of nanorefrigerants are mass flux and vapor quality.
The research reported in the literature focuses on the analysis of heat transfer single phase nanofluids, and some reference pool nucleate boiling. However, the research on phase change in flow boiling of nanofluids, which are very common in refrigeration systems, heat exchangers and air conditioners, is very limited. Boiling characteristics and nanofluids flow in two phases dependent on properties such as specific heat, latent heat, density, surface tension, to name but a few [16] . Further research is needed to enable a better understanding of the properties of nanofluids and how to measure them in evaporation and condensation systems for application in a refrigeration cycle.
Mahbubul et al. [17] conducted a study of the characteristics of heat transfer and pressure drop to a mixture of Al2O3-R141b for different concentrations and phase change due to flow boiling. The analysis was performed in a pipe of 6 mm of diameter and 1000 mm of length. The analysis was undertaken with an inlet temperature of 298 K, a pressure of 78,535 kPa, a mass flux of 100 kg/m2.s and an input speed of 5 m/s, resulting in a turbulent flow through the pipe. The wall was subjected to a uniform heat flux of 5000 W/m2. They used a correlation given by [14] to calculate the heat transfer coefficient. They found that both heat transfer and pressure drop increase with increasing volume concentration of nanoparticles, which can improve the performance of cooling systems by increasing energy efficiency and cooling capacity.
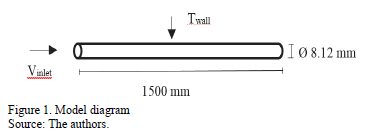
In a subsequent study, Mahbubul et al. [18] investigated the thermophysical properties, pressure drop and heat transfer of 30 nm Al2O3 nanoparticles suspended in the R-134a refrigerant. Nanoparticle concentration varied from 1 vf% to 5 vf% [17] . The pipe used for analysis was 8. 12 mm of diameter and 1500 mm of length. Entry conditions were 706 kPa, 300 K, uniform mass flux of 100 kg/m2s, 5 m/s of velocity and a uniform wall heat flow rate of 5000 W/m2. In the research presented in this paper, these data were taken as a reference for the analysis of different nanorefrigerants, but using a constant wall temperature of 330 K and a temperature of 298.15 K at the inlet of the pipe.
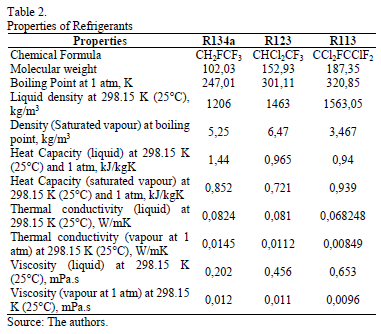
Simulations were carried out in ANSYS FLUENT 15.0 varying the working fluid (R113, R123 and R134a), with 0 vf.%, 1 vf.% and 5 vf.% of Al2O3 nanoparticle concentration. Two cases were studied, at 0.5 m/s and 5 m/s (Reynolds number for R134a of 24239.40 and 242394.0 respectively) inlet velocity in a horizontal tube with a constant wall temperature. Results were compared taking into account the change in pressure drop, temperature through the tube, heat transfer coefficient and thermal conductivity between the base fluid and the nanofluid. The properties of the nanoparticles and refrigerants are shown in Table 1 and 2 respectively, these are considered constant for the study.
In [19] and [20] the properties of the nanofluid were obtained before the simulation because it was taken as a single phase model. For this reason, the Brownian effects were taken into account in order to calculate the effective thermal conductivity. In the present study, the nanofluid has a change of phase from liquid to vapor, and it is considered to be a multiphase model where particles and fluid were solved using a mixture model. "The mixture model solves the continuity equation for the mixture, the momentum equation for the mixture, the energy equation for the mixture, and the volume fraction equation for the secondary phases, as well as algebraic expressions for the relative velocities (if the phases are moving at different velocities)" [21] . The effective thermal conductivity is calculated by Fluent as a summatory of the thermal conductivity of each phase plus the turbulent thermal conductivity given by the turbulent model used.
2. Mathematical models
Two approaches have been reported in the literature to model dynamic phenomena and heat transfer of nanofluids [10] . The first approach treats nanofluids as a single phase fluid, assuming that the solid particles are in thermal equilibrium with the fluid phase and the relative velocity between them is zero. The second approach found in the literature adopted a two-phase flow, where the moving speed between the particles and the fluid is not necessarily zero. Authors argue that simulations that treat the solid phase and liquid separately have more accurate results when seen as a single phase. The most appropriate used model to simulate the flow of the two phases in CFD is the mixture model.
Akbari et al. in [22] developed a computational study of convection with a mixture of water and Al2O3 nanoparticles in a horizontal pipe. The analysis was carried out with uniform heat flow to compare the single-phase model, and the three CFD models for multiphase flow (Volume of Fluid, Mixture and Eulerian). The authors found very similar results for the hydrodynamic behavior of nanofluid. However, the thermodynamic results differ in the different models used; the two-phase model being more accurate. For successful results with the single phase model it is necessary to use complex correlations to effectively characterize the properties of the nanofluid.
There are two approaches for using the two phase model. The Lagrangian-Eulerian approach, which is used when the volume fraction of the secondary phase is less than 10 vf.% and the Eulerian-Eulerian approach for higher volume fractions. Generally the amount of nanoparticles used in nanofluids is very large even for very small volume fractions; therefore, it is not advisable to use the Lagrangian-Eulerian approach due to the high computational cost that this implies [22] . Moraveji et al. in [23] made a comparison between CFD models of one and two-phases to study heat transfer by flow boiling Al 2O3-water with 100 nm nanoparticles, in a pipe with constant heat flux on the wall. Heat transfer is enhanced by increasing the concentration of nanoparticles and the Reynolds number. Numerical data were obtained using a correlation for the Nusselt number in a horizontal line based on the Reynolds (Re) and Prandtl (Pr) numbers and the volume fraction (j) [23] .
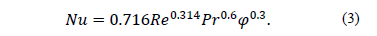
The subcooled flow boiling of Al2O3-water with 30 nm nanoparticles was investigated numerically by Abedini et al. in [24] . The nanoparticles have a large effect on the thermophysical properties of the base fluid, such as viscosity, specific heat and density. In this case it is assumed that a nanofluid behaves as a homogeneous liquid due to the low volume fraction of vapor during subcooled boiling, so the system is solved by a two-phase model, the phase of nanofluid and the vapor phase [24] . A Mixture Eulerian model is used for the computational solution because it represents a simple way to solve the problem by obtaining a coefficient of heat transfer consistent with experimental data. The results show that increasing the concentration of nanoparticles increases the heat transfer. However, low concentrations of particles (1 - 2 vf.%) are more effective in heat transfer coefficient.
ANSYS Fluent has the ability to solve problems with multiphase flows, which are grouped into four categories: gas-liquid or liquid-liquid flow; gas-solid flow; liquid-solid flow; and three-phase flows [21] .
The Mixture Model treats phases as interpenetrating continuous phases [25] . It solves the momentum, continuity, and energy equations for the mixture, and solves the equation of volume fraction for the secondary phases and prescribes relative velocities to describe the dispersed phases through algebraic expressions.
The Mixture Model selects granular phases and calculates their properties for application in liquid-solid flows. It also allows the phases move at different velocities using the concept of sliding velocities. The Mixture and the k-e turbulent models were employed to obtain results for the heat transfer coefficient and temperature, and the Coupled algorithm was chosen for the pressure-velocity coupling, which solves all equations for phase velocity corrections and shared pressure correction simultaneously [21] .
The continuity equation for the mixture is:
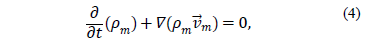
where 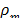 is the density of the mixture and
is the density of the mixture and 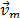 is the averaged flow velocity:
is the averaged flow velocity:
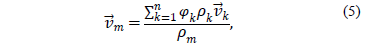
where the subindex 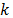 represents the phases of the mixture.
represents the phases of the mixture.
The mixture density is defined by:
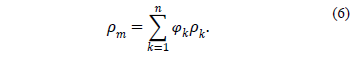
The momentum equation for the mixture is obtained by adding momentum equations for each phase
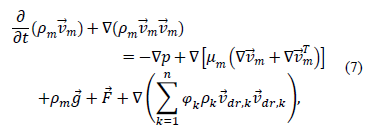
where 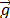 is the acceleration of gravity,
is the acceleration of gravity, 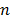 is the number of phases,
is the number of phases, 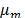 is the viscosity of the mixture and is defined by:
is the viscosity of the mixture and is defined by:
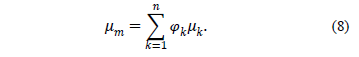
The drift velocity of the second phase 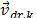 is defined by:
is defined by:
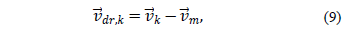
The energy equation is applied as follows:
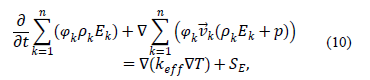
where 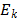 is the energy of each phase,
is the energy of each phase, 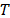 is the temperature,
is the temperature, 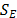 includes others volumetric heat sources, and
includes others volumetric heat sources, and 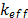 is the effective thermal conductivity, expressed as follows:
is the effective thermal conductivity, expressed as follows:
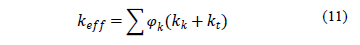
where 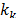 represents the thermal conductivity of each phase of the mixture and
represents the thermal conductivity of each phase of the mixture and 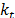 is the turbulent thermal conductivity defined by the turbulent model used.
is the turbulent thermal conductivity defined by the turbulent model used.
From the continuity equation for phase p, it can be can obtained the volume fraction equation of the secondary phase:
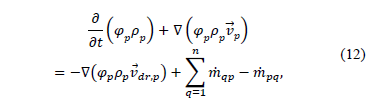
where 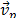 is the velocity of phase p and
is the velocity of phase p and 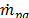 characterizes the mass transfer from the pth to qth phase.
characterizes the mass transfer from the pth to qth phase.
3 Results and discussion
For the simulations, different meshes were used to validate the accuracy and consistency of the results, refining the mesh and comparing the heat transfer coefficient for each case. After a mesh independence study a mesh of 1'072.768 elements was selected because the results of the heat transfer coefficient do not change significantly with the mesh.
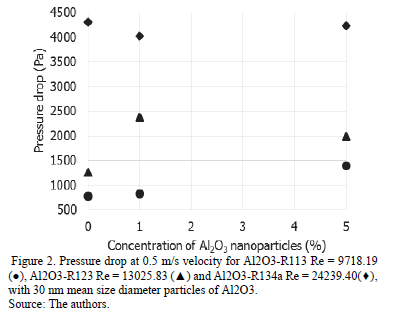
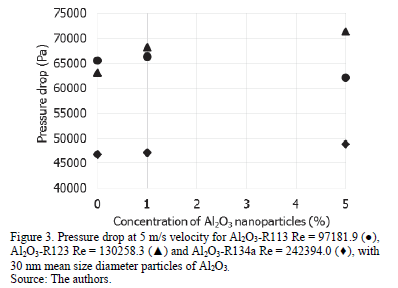
The results cannot determine the influence of the concentration of particles in the pressure drop of the pipe since the behavior is different in each case as shown in Fig. 2 and 3, where the pressure drop is presented for the three nanorefrigerants analyzed at different concentrations of nanoparticles with a mean size diameter of 30 nm, and a different Reynolds number.
The pressure drop for simulations with an initial velocity of 0.5 m/s does not show a pattern that determines the influence of the size and concentration of nanoparticles. In this case, pressure drop for nanorefrigerant R134a and 30nm Al2O3 at 1vf% is 4022.5 Pa. By increasing the initial velocity at 5m/s, the pressure drop increases significantly, but is not affected by the size or by the concentration of particles. For nanorefrigerant R134a and 30nm Al2O3 at 1vf% at v=5m/s, the pressure drop is 47071.14 Pa.
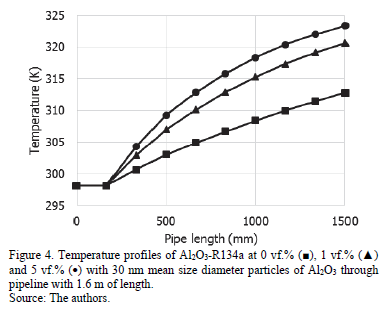
An increase in the temperature of the fluid along the pipe is observed by adding nanoparticles and the improvement is greater by increasing nanoparticle concentration as shown in Fig. 4. At the beginning of the pipe, the temperature of the three fluids is very similar, until 0.2 m when it starts to increase. The maximum values are obtained with a 5vf% nanoparticles concentration.
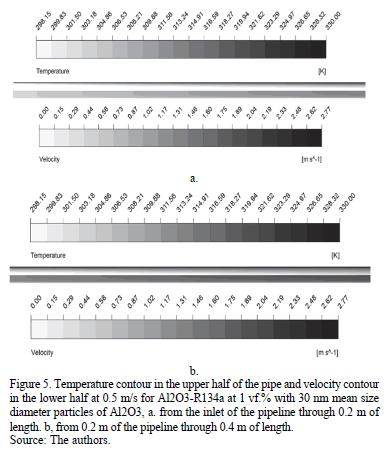
In Fig. 5a. the temperature contour in the upper half of the pipe and the velocity contour in the lower half, from the inlet through 0.2 m of length are shown, in order to compare the thermal and the hydrodynamic boundary layers. This is possible because of the symmetry of the problem. It can be observed that the thermal boundary layer develops more slowly than the hydrodynamic layer, which is why there is not a significant change in temperature in the first 0.2 m of length.
The temperature contour in the upper half of the pipe and the velocity contour in the lower half, from 0.2 m through 0.4 m of the pipe is shown in Fig. 5b., where the temperature starts to increase while the hydrodynamic boundary layer is nearly fully developed.
In addition, the influence of particle size on temperature and pressure drop was evaluated throughout the pipeline. It was observed that where the mean size of particle is 20 and 50 nm the temperature had the same behavior as in Fig. 4 where the particle size is 30 nm. Therefore, particle size does not have a significant impact on the temperature of the nanorefrigerant.
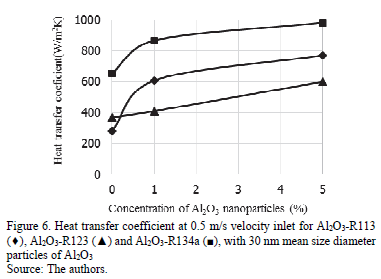
The heat transfer coefficient is presented in Fig.6. where a comparison of the three different nanorefrigerants is made at a 0.5 m/s velocity inlet with a 30 nm mean size diameter at 1 vf.% and 5 vf%. R134a has the greatest heat transfer coefficient at these conditions. Al2O3-R134a with 1 vf.% show a 24.60 % increase in the heat transfer coefficient in relation to the pure refrigerant, but only 11.73 % more with a concentration of 5 vf.% of particles. Al2O3-R113 shows an increase of 54.05 % of the heat transfer coefficient by adding 1 vf.% of nanoparticles to the pure refrigerant, and an increase of 21.16 % with 5 vf.% of nanoparticles. And Al2O3-R123 has a more linear behavior with a 9.87 % increase for 1 vf.% of nanoparticles in relation to the pure refrigerant and an increase of 31.62 % of the heat transfer coefficient with 5 vf.% of Al2O3.
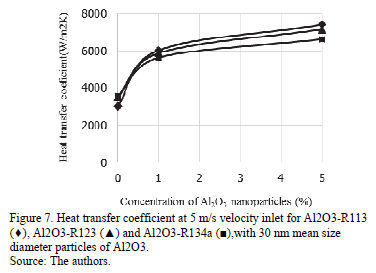
Source: The authors. Increasing the velocity inlet up to 5 m/s causes higher values of heat transfer coefficient, 3000 W/m2K approximately, for base refrigerants. Fig. 7. shows a similar behavior of the heat transfer coefficient for 1 vf.% and 5 vf.% of nanoparticles. Adding 1 vf.% of Al2O3 to any of the studied refrigerants increases the heat transfer coefficient to 41 % approximately, and 17 % more by adding 5 % of nanoparticles, for a total of 58 % in relation to the pure refrigerant. The heat transfer coefficient was improved by increasing the nanoparticle concentration. However, the improvement with 5 vf.% of nanoparticles is not significant enough, and 1 vf.% of Al2O3 is adequate for further calculations.
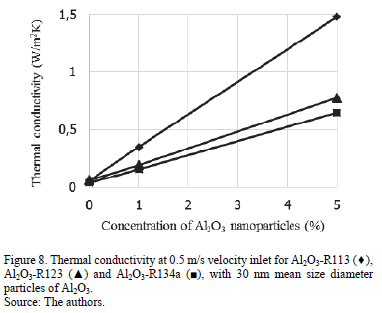
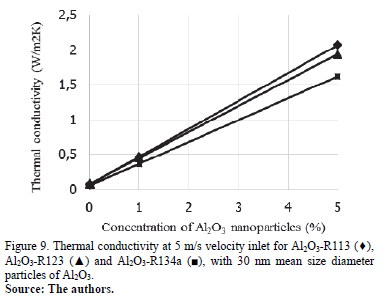
Thermal conductivity in relation to the concentration of nanoparticles is presented in Fig. 8 and Fig. 9 at 0.5 m/s and 5 m/s respectively. These graphics show an increase in thermal conductivity by adding nanoparticles at different concentrations. Al2O3 nanoparticles has a great impact on R113 thermal conductivity at 0.5 m/s, while R123 and R134a have a lower thermal conductivity and a very similar tendency to increase. At 5 m/s the thermal conductivity of nanorefrigerants can have a very similar increase by adding more particles. In this example, particle size has no influence on thermal conductivity behavior.
Results show that adding nanoparticles to refrigerant fluids improves the thermal characteristics such as thermal conductivity and the heat transfer coefficient, which could mean enhancing the performance of refrigeration systems. The pressure drop of nanofluids studied does not show a particular relation with respect to the nano particles concentration, not allowing to conclude about the effect of that variable on the pressure drop, that is why further research efforts should analyze this feature as well as the migration of particles due to phase change since the mixture model is unable to predict it correctly.
It was observed that nanoparticle size does not affect the thermal characteristics of nanofluids. On the other hand, a concentration of nanoparticles results in an improvement to those characteristics, 1 vf.% being the best concentration because it has a significant impact on thermal conductivity and the heat transfer coefficient with less particles than 5 vf.% concentration.
Results show an improvement in thermal characteristics by increasing inlet velocity for each of the refrigerants, however this means a significant increase in pressure drop. At a higher inlet velocity, the difference in thermal conductivity and in heat transfer coefficient is not appreciable between the three studied nanorefrigerants.
R134a with 30 nm Al2O3 at 1 vf.% is a excellent option because it has a lower environmental impact and it has an exceptional thermal performance which is convenient in refrigerant systems.
Acknowledges
This work was funded by Colciencias through the Young Research Program under grant 617-2013.
References
[1] Dinçer, I. and Kanoglu, M., Refrigeration systems and applications, Second Ed., United Kingdom: John Wiley & Sons, Ltd, 2010. [ Links ]
[2] McLinden, M.O., Kazakov, A.F., Brown J.S. and Domanski, P.A., A thermodynamic analysis of refrigerants: Possibilities and tradeoffs for Low-GWP refrigerants. International Journal of Refrigeration, 38, pp. 80-92, 2014. DOI: 10.1016/j.ijrefrig.2013.09.032. [ Links ]
[3] Sarbu, I., A review on substitution strategy of non-ecological refrigerants from vapour compression-based refrigeration, air-conditioning and heat pump systems. International Journal of Refrigeration, 46, pp. 124-141, 2014. DOI: 10.1016/j.ijrefrig.2014.04.023. [ Links ]
[4] Domanski, P.A., Brown, J., Heo, S.J., Wojtusiak, J. and McLinden, M.O., A thermodynamic analysis of refrigerants: Performance limits of the vapor compression cycle. Int Journal of Refrigeration, 38, pp. 71-79, 2014. DOI: 10.1016/j.ijrefrig.2013.09.036. [ Links ]
[5] Vélez, F., Selecting working fluids in an organic rankine cycle for power generation from low temperature heat sources, DYNA, 81(188), pp. 173-180, 2014. DOI: 10.15446/dyna.v81n188.41666. [ Links ]
[6] Calm, J.M. and Didion, D.A., Trade-offs in refrigerant selections: Past, present, and future, International Journal of Refrigeration, 21(4), pp. 308-321, 1998. [ Links ]
[7] Basaran, A. and Ozgener, L., Investigation of the effect of different refrigerants on performances of binary geothermal power plants. Energy Conversion and Management, 76, pp. 483-498, 2013. DOI: 10.1016/j.enconman.2013.07.058. [ Links ]
[8] Saidur, R., Leong, K. and Mohammad, H., A review on applications and challenges of nanofluids, Renewable and Sustainable Energy Reviews, 15, pp. 1646-1668, 2011. DOI: 10.1016/j.rser.2010.11.035. [ Links ]
[9] Wong, K.V. and De León, O., Applications of nanofluids: current and future. Advances in Mechanical Engineering, 2010. DOI: 10.1155/2010/519659. [ Links ]
[10] Kamyar, A., Saidur, R. and Hasanuzzaman, M. Application of Computational Fluid Dynamics (CFD) for nanofluids," International Journal of Heat and Mass Transfer, 55, pp. 4104-4115, 2012. DOI: 10.1016/j.ijheatmasstransfer.2012.03.052. [ Links ]
[11] Choi, S.U.S.D.W.H.e., Development and application of non-Newtonian flows, First ed., 231, in: Wang, D.A.S.a.H.P. Ed., ASME Press, New York, 1995, pp. 99-105. [ Links ]
[12] Saidur, R., Kazi, S., Hossain, M., Rahman, M. and Mohammed, H., A review on the performance of nanoparticles suspended with refrigerants and lubricating oils in refrigeration systems, Renewable and Sustainable Energy Reviews, 15, pp. 310-323, 2011. DOI: 10.1016/j.rser.2010.08.018. [ Links ]
[13] Peng, H., Ding, G., Hu, H. and Jiang, W., Effect of nanoparticle size on nucleate pool boiling heat transfer of refrigerant/oil mixture with nanoparticles. International Journal of Heat and Mass Transfer, 54, pp. 1839-1850, 2011. DOI. 10.1016/j.ijheatmasstransfer.2010.12.035. [ Links ]
[14] Peng, H., Ding, G., Jiang, W., Hu., H. and Gao, Y., Heat transfer characteristics of refrigerant-based nanofluid flow boiling inside a horizontal smooth tube. International Journal of Refrigerant, 32, pp. 1259-1270, 2009. DOI: 10.1016/j.ijrefrig.2009.01.025. [ Links ]
[15] Sun, B. and Yang, D., Flow boiling heat transfer characteristics of nano-refrigerants in a horizontal tube, 38, 2014. DOI: 10.1016/j.ijrefrig.2013.08.020, pp. 206-214. [ Links ]
[16] Cheng, L. and Liu, L., Boiling and two-phase phenomena of refrigerant-based nanofluids: Fundamentals, applications and challenges, International Journal of Refrigeration, 36, pp. 421-446, 2013. DOI: 10.1016/j.ijrefrig.2012.11.010. [ Links ]
[17] Mahbubul, I., Saidur, R. and Amalina, M., Heat transfer and pressure drop characteristics of Al2O3-R141b nanorefrigerant in horizontal smooth circular tube, Procedia Engineering, 56, pp. 323-329, 2013. DOI: 10.1016/j.proeng.2013.03.126. [ Links ]
[18] Mahbubul, I., Fadhilah, S., Saidur, R., Leong, K. and Amalina, M., Thermophysical properties and heat transfer performace of Al2O3/R-134a nanorefrigerants. International Journal of Heat and Mass Transfer, 57, pp. 100-108, 2013. DOI: 10.1016/j.ijheatmasstransfer.2012.10.007. [ Links ]
[19] Togun, H. Ahmadi, G., Abdulrazzaq, T., Shkarah J.A., Kazi, S.N., Badarudin, A. and Safaei, M.R., Thermal performance of nanofluid in ducts with double, Journal of the Taiwan Institute of Chemical Engineers, vol. 47, pp. 28-42, 2015. DOI: 10. [ Links ]1016/j.jtice.2014.10.009.
[20] Yarmand, H., Gharehkhani, S., Newaz-Kazi, S., Sadeghinezhad E. and Reza-Safaei, M., Numerical investigation of heat transfer enhancement. The Scientific World Journal, 2014, Article ID 369593, 9 P., 2014. DOI: 10.1155/2014/369593. [ Links ]
[21] ANSYS, Inc, Ansys fluent 12.0 Theory Guide, ANSYS, Inc, 2009. [ Links ]
[22] Akbari, M., Galanis, N. and Behzadmehr, A., Comparative analysis of single and two-phase models for CFD studies of nanofluid heat transfer, International Journal of Thermal Sciences, 50(8), pp. 1343-1354, 2011. DOI: 10.1016/j.ijthermalsci.2011.03.008. [ Links ]
[23] Moraveji, M.K. and Esmaeili, E., Comparison between single-phase and two-phase CFD modeling of laminar forced convection flow of nanofluids in a circular tube under constant heat flux. International Communications in Heat and Mass Transfer, 39(8), pp. 1297-1302, 2012. DOI: 10.1016/j.icheatmasstransfer.2012.07.012. [ Links ]
[24] Abedini, E., Behzadmehr, A., Sarvari, S. and Mansouri, S., Numerical investigation of subcooled flow boiling of a nanofluid. International Journal of Thermal Sciences, 64, pp. 232-239, 2013. DOI: 10.1016/j.ijthermalsci.2012.08.008. [ Links ]
[25] Davarnejad, R. and Jamshidzadeh, M., CFD modeling of heat transfer performance of MgO-water nanofluid under turbulent flow, Engineering Science and Technology, International Journal, 18(4), pp. 536-542, 2015. DOI: 10.1016/j.jestch.2015.03.011. [ Links ]
[26] Betancur-Márquez, S., Alzate-Espinosa, G.A. and Cortés-Correa, F.B., Mejoramiento de los fluidos de perforación usando nanopartículas funcionalizadas. Boletín Ciencias de la Tierra, 35, pp. 5-14, 2014. DOI: 10.15446/rbct.n35.43179. [ Links ]
D.C. Hernández, received her BSc. Eng in Aeronautical Engineering in 2014, from the Universidad Pontificia Bolivariana. Medellin, Colombia. Currently, she is a young researcher at the Research group on Energy and Thermodynamic of Universidad Pontificia Bolivariana. Her research interests include: computer simulation, modeling and forecasting in heat transfer, and fluid dynamics. ORCID: 0000-0002-0252-687X
C. Nieto-Londoño, received his BSc. Eng in Mechanical Engineering in 2003, his MSc. degree in Energy in 2006, and a Dr. degree in Engineering - Energy Area in 2012. He works on projects of numerical simulation applied to the solution, evaluation and design of several applications, including micro and nano fludis, renewable energy, micro combustion, catalytic fluid and porous media, for the Universidad Pontificia Bolivariana, Medellín, Colombia. He is currently coordinator of the Doctorate in Engineering and a researcher on the Energy and Thermodynamic Group and the Engineering Aerospace Research Group, at the Universidad Pontificia Bolivariana. ORCID: 0000-0001-6516-9630
Z. Zapata-Benabithe, received her BSc. Eng in Chemical Engineering in 2003, her MSc. degree in Energetic Systems in 2008, and a Dr. degree in Engineering - Energy Area in 2014. She works on projects of gasification, pyrolysis and carbon materials for electrochemical energy storage, for the Universidad Pontificia Bolivariana, Medellín, Colombia. She is currently coordinator of the Thermofluids and Energy Conversion Seedbed of the Energy and Thermodynamic Group, at the Universidad Pontificia Bolivariana. ORCID: 0000-0002-4497-4865.