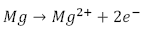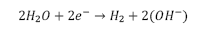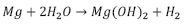1. Introduction
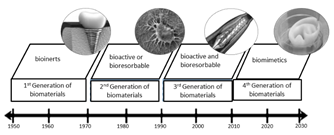
Biomaterials are either natural or synthetic materials used to replace or assist an organ or tissue. They are in direct contact with biological systems [1] and they must be biocompatible. Biocompatibility was defined in 2008 as "the ability of a material to perform the required function in response to medical therapy without causing undesired effects at either the local or systemic level in a user. The material must also produce a proper cell or tissue response in order to create relevant clinical therapy" [2]. Moreover, a biocompatible material can be inert or can interact and react with the tissue. Other biocompatible materials are degraded by the body over time, and the adaptation of the material response to the body is decisive. Biocompatibility then assesses the biological activity of the material after being in contact with human tissue. Accordingly, a biocompatible material must not be genotoxic, cytotoxic, mutagenic, carcinogenic, or immunogenic [3]. Biomaterials have been developed in four different generations, and they are grouped according to clinical requirements. Inert biomaterials have been developed and selected since 1950 to enhance repair processes or replacement of the host tissue without causing an adverse reaction in the biological system; these materials were called first-generation biomaterials. They included cobalt alloys, alumina, and stable polyurethane and had great importance in orthopedic and dental applications.
The second generation of biomaterials started due to poor adhesion and implant loosening, which called for the development and use of materials with bioactive surfaces capable of forming complex bonds with the tissue. At the same time, the use of biodegradable materials was also being researched; thus, since 1970, materials with bioactive or biodegradable properties have been studied. Titanium and calcium phosphates, including hydroxyapatite, are examples of second-generation biomaterials. However, the materials mentioned above showed limited durability over time. Further research is based on finding temporary materials capable of being degraded in a controlled manner over time and promoting a natural integration of the implant with the tissue that they should eventually replace. Bioactivity and biodegradation properties of third-generation materials such as magnesium and its alloys are currently under study for tissue regeneration and cardiovascular stents [1]. Since around 2010, researchers began studying a new generation of biomaterials. This latest generation of biomaterials encompasses biomimetic nanocomposites for applications in tissue engineering [4]. Fig. 1. shows the four generations of biomaterials [5].
Currently, biomaterials science is focused on the development of temporary degradable structures allowing tissue integration with the implant, in order to improve the replacement or repair process. After repair, the implant is degraded and it is replaced by host tissue. Consequently, degradable biomaterials for bone repair are generating much interest -because they can save additional implant removal procedures, reducing pain and health care costs.
On one hand, biodegradable or resorbable polymers such as poly(lactic-co-glycolic acid) (PLGA), polylactic acid (PLA), and polyglycolic acid (PGA) are currently being clinically applied. However, they have shown adverse effects in the human body when the immune system triggers osteolytic reactions around the implant after a foreign body -for example, a polymer- is recognized [6,7].
On the other hand, magnesium alloys are a new class of promising materials currently under development [8]. Magnesium alloys exhibit poorer corrosion resistance in Cl--containing physiologic environments. Thus, these alloys could be used as biodegradable metals. Moreover, magnesium is essential to human metabolism, and it is the fourth most abundant cation in the human body [9].
Biodegradable magnesium-based materials usually have a high corrosion or biodegradation rate. Since this degradation occurs in the early stages of implantation [10,11], this is a limitation for using it in biomedical implants. The high degradation rate of Mg presents several problems: 1. Production of subcutaneous hydrogen bubbles during the corrosion of the metal can cause accumulation of hydrogen around the implant. Hydrogen can affect the healing process and may lead to tissue necrosis due to formation of gaps between the implant and the tissue [10,12,13]. 2. Changes in local pH values caused by generation of OH- anions during the cathodic reaction of the corrosion process [14]. This process affects some cellular functions and cell viability [10]. 3. The high corrosion rate of magnesium also produces an early degradation of transverse sections of the implants. Due to the fact that the time required by the bone to regenerate tissue is between 3 and 4 months approximately. During that time the implant must maintain mechanical integrity [15]. Nevertheless, Mg implants will not withstand the mechanical stresses to perform osseointegration if they are corroded. In the literature, some authors have reported that the magnesium degradation period is shorter than the period required to achieve mechanical integrity [16]. Still, the time for the implant to be stable depends on several factors, such as type of study (in vivo and in vitro studies), material composition, implant location, type of model (different animals), and days of evaluation of the in-vivo tests. Moreover, since several methods are available for measuring degradation rates, it is difficult to obtain correlations with in vitro tests. As a result, nowadays there is great interest in the surface properties of magnesium and its alloys to achieve enhanced corrosion resistance and biocompatibility while retaining the bulk properties of the material [17].
The corrosion rate of magnesium and magnesium alloys is a key factor for the development of new biomaterials to be used in resorbable implants. Therefore, coatings have emerged as a possible solution, enabling the reduction of degradation at the early stages of implanted materials and positively affecting the tissue recovery process. Surface modification of magnesium can be implemented to prevent electrolytes in the biological medium or in electrolyte solutions from degrading the material quickly. Titanium dioxide is a ceramic that has been widely used as a coating material, mainly because it improves the corrosion resistance of the surface-covering material (generally metals and alloys) [18,21]. Although magnesium is a bio-inert material, several authors have argued that it favors the formation of calcium phosphates on its surface, which gives the material bioactive properties that can be used in bone applications [22,23]. Furthermore, the photocatalytic activity of titanium dioxide provides antibacterial properties [24].
This paper reviews magnesium modified with Titanium dioxide, but it does not focus on the techniques since there are several reviews already available in the literature [11,13,25]. Its main goal is to gather information concerning the development of titanium coatings to improve the properties (corrosion rate and biocompatibility) of magnesium for applications in biomaterials. In addition, the importance of sol-gel techniques for biomedical coatings is highlighted.
2. Magnesium
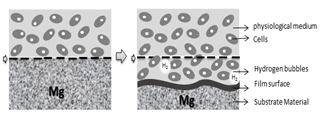
Magnesium is a metal of interest for bone applications due to the fact that its density (1.74 g / cm3) and its Young’s modulus of 45 GPa are very similar to those of human bone (1.8-2.1 g / cm3 and 40-57 GPa respectively). Magnesium is also very abundant in the human body, and it is essential for cells, mainly because it takes part in various metabolic processes inside the body. Additionally, it is known as an essential ion since it participates in the formation of apatite in the bone matrix. Magnesium implants were used for the first time in 1878, but it was not until 1990 that they were used again in orthopedic applications, when Erwin Payr [26] introduced fixator pins, pegs, wires, and nails based on biodegradable Mg for treatments on bone fractures. When magnesium alloys are used for orthopedic implants, they provide adequate mechanical properties, low stress shielding effects (to avoid bone resorption), good biocompatibility, and a controlled degradation rate [27]. However, interest in implants made of magnesium and its alloys gradually decreased for a period of time, because they are highly susceptible to rapid corrosion in physiological environments, limiting their application [28]. This accelerated corrosion leads to premature failure of implants, since the support needed for tissue healing is not provided. Furthermore, production of large amounts of subcutaneous hydrogen bubbles and modification of local pH could eventually lead to tissue necrosis, as it was shown in a review by Frank Witte [28]. Fig. 2. presents the natural process of degradation of magnesium in a physiological environment [5].
Generally speaking, corrosion of magnesium occurs in aqueous solutions and various redox reactions are present, depending on the alloying elements. It is known that hydrogen and magnesium hydroxide will appear during the process. The most common corrosion reactions of Mg are shown in eq. (1-3) [30].
Anodic: Dissolution of metal
Cathodic: Evolution of hydrogen
Net reaction:
Given that magnesium is (electrochemically) one of the most active metals and that it consequently has high corrosion rates, several alloys have been developed to overcome those issues and promote its use in numerous applications. Therefore, magnesium alloys are part of the third generation of biomaterials and they can be classified in different families: Mg-Al, Mg-Ca, Mg-Sr, Mg-Zn, Mg-Si, Mg-Sn, Mg-Mn, Mg-Re, and Mg-Ag [31,32]. The most commonly used commercial alloys are AZ (Mg-Al-Mn), AM (Mg-Al-Mn), AE (Mg-Al-Re), the EZ series (Mg-Zn-Re), ZK (mg-Zn-Zr), WE (Mg-RE-Zr), or AX AXJ (Mg-Al-Ca), and the AJ series (Mg-Al-Sr) [33]. Different alloys have particular advantages for certain applications and their properties have been extensively evaluated. The literature reports that the corrosion rate increases as temperature increases and it decreases with aluminum content [34, 35]. Pardo et al. [36] assessed the corrosion behavior of commercial alloys AZ31 (3.1% Al, 0.73% Zn, 0.25% Mn), AZ80 (8.2% Al, 0.46% Zn, 0.13% Mn), and AZ91D (8.8% Al, 0.68% Zn, 0.30% Mn). The authors concluded that an increase in aluminum concentration significantly reduces the corrosive activity of pure magnesium (99%). They also determined that the AZ31 alloy is more corrosion-resistant in a solution of sodium chloride (NaCl).
Since 2000, multiple original papers and reviews have reported that magnesium has no adverse effects on the human body -mainly because Mg is a trace element of the human body- and the corrosion products can be considered physiologically beneficial. Yun et al. [37] evaluated the biocompatibility of Mg under corrosion effects in osteoblasts. The mineralization and cytotoxicity results were not significantly affected by the presence of corroded Mg. In a comparative study by Witte et al. [28], four commercial alloys - AZ31, AZ91, WE43, and LAE442- were implanted in guinea pig femurs for 6 and 18 weeks, demonstrating the osteoinductive capacity of Mg-based materials. Recently, Zhen et al. [38] used the murine fibroblasts cell line (L929) to test cell viability in different concentrations of Mg2+ at various pH values in a DMEM medium, and the results show that there is a considerable hemolysis rate. However, the result was found for pH values higher than 11, which is above the average physiological pH (pH = 7). Additionally, the viability of cells treated with low concentrations of Mg2+ (<100 µg / mL) is above 80%, showing no significant toxicity to L929 cells.
Currently, the possible medical applications of magnesium have gained more attention [31]. Therefore, magnesium has been used for the manufacture of biodegradable stents [39,40], in order to avoid diseases such as thrombosis and restenosis via drug delivery [41]. Great efforts to study biodegradable magnesium -in the form of implants for orthopedic applications- are focused on improving the corrosion resistance by surface modification in order to provide mechanical stability to the material. This is done by favoring the initial attachment and subsequent degradation that assist the cicatrization processes.
3. Coatings for magnesium implants
The surfaces of biomaterials implanted in the human body are very important because they are in direct contact with host tissue. Additionally, since reactions take place at the interface, the capability of the material to be used as biomaterial is determined by its surface.
Due to the fact that most materials are not biocompatible, several methods have been used to modify their surface properties, in order to improve biocompatibility, biofunctionality, topography, and physical-chemical properties, enabling the materials to maintain their bulk material properties. That can be achieved with surface modification, integration, and mechanical attachment to host tissue [25]. Besides, the modified surface can be an effective barrier against the physiological environment.
Coatings emerge as possible solutions to highly corrosive media, forming films or layers on materials (ceramic or polymeric materials). A study by Tang et al. [42] compared in-vitro and in-vivo Mg-Zr coatings obtained by micro arc oxidation (MAO) and electrophoretic deposition (EPD). The authors established that EPD coatings are more corrosion-resistant than coatings obtained by MAO. The results were attributed to differences in the coating structure, mostly because cracks or pores were not observed on the surface of EPD coatings, while micropores were fully distributed on the surface of MAO coatings. However, the cell-material interactions were not considered in that study. Although the results are satisfactory, the insufficient adhesion of coatings is the main obstacle of the EPD technique and its potential application. Subsequent studies [43] proposed a coating method for the micro arc oxidation and liquid phase deposition in order to obtain bioactive coatings on a Mg-Zn-Ca alloy. The authors stated that an evident improvement was obtained in the substrate corrosion resistance, although the coating obtained by MAO was porous.
Several polymers have been used as coatings because they enhance the corrosion rate of magnesium and also have acceptable biocompatibility. Witecka et al. [44] reported that four different biodegradable polymers (PLGA, PLLA, PHBV, and PHB) on Mg-2.0Zn-0.98Mn magnesium alloys obtained by spin-coating successfully reduced the corrosion of the substrate. Likewise, the results indicated that both cytocompatibility and cell functionality were improved. Ma et al. [45] have reviewed polymeric coatings for magnesium and magnesium alloys and gathered additional information.
Ceramic coatings have also been successfully produced. On one hand, Ren et al. [46] used a microwave-assisted coating process to obtain a ceramic coating (HA and calcium deficient hydroxyapatite -CDHA) on a AZ31 magnesium alloy substrate. The authors fabricated uniform coatings in less than 10 minutes, and the results showed that those coatings improved corrosion behavior, they had good cytocompatibility and promoted cellular proliferation after 5 days of incubation in a cell culture medium. On the other hand, ceramic coatings were fabricated by micro arc oxidation (MAO) on ZK61 magnesium alloys by Yu et al [47]. The authors conclude that corrosion resistance was improved by the coating, and that the latter favored the precipitation of bone-like apatite on the coating surface after immersed in SBF for 14 days.
Other authors have studied nanostructured coatings on magnesium alloys for biomedical applications. Recently, a novel bi-layered nanostructured SiO2/Ag-FHAp coating on biodegradable magnesium has been successfully synthesized by physical vapor deposition (PVD) combined with electrodeposition (ED). The results showed high corrosion resistance and biocompatibility [48].
3.1. Magnesium coated with titanium dioxide
Mechanical and biological fixation to the surrounding bone is one of the major concerns regarding orthopedic implants. Therefore, current research is focused on surface modifications to improve the material’s osteoconductivity, to have it act as a structural support and to promote new bone formation and growth. Osteoconductivity must be complemented with biodegradability because implant materials must be reabsorbed or biodegraded into the body, in order to enable the new tissue to grow and to provide an adequate support [49]. Some calcium phosphates (CaP) -including hydroxyapatite (HA)- have been proposed to improve osseointegration of metal implants [50]. However, titanium dioxide has emerged as a potential candidate to improve the magnesium surface properties and corrosion resistance.
Titanium dioxide (TiO2) and titanium oxide (IV) are metal oxides. TiO2 crystallizes naturally in three main forms: rutile, anatase, and brookite. The structure of this oxide is based on a titanium atom surrounded by six oxygen atoms in a distorted octahedral configuration [51]. Titanium oxide is stable and non-toxic to the environment or humans. It also has high catalytic activity, high oxidizing power, and it is stable against photocorrosion [52]. Furthermore, it presents antibacterial properties [24]. Different authors have reported that titanium oxide induces the precipitation of bone-like apatite or calcium phosphates on its surface [53-55]. Those properties make titanium dioxide a suitable candidate for bone replacement and reconstruction.
Coatings with titanium dioxide are well known to have antibacterial properties. Guo et al. [56] observed the biocidal effect of a porous coating with nanoparticles containing Ag and TiO2, obtained by a sol-gel route. Fu and coworkers [57] observed the biocidal effect as well when using titanium dioxide coatings obtained by sol-gel methods on glass substrates. In general, the antibacterial effect of TiO2 has been extensively researched and the biological mechanism by which this happens is currently under study [24,58]
Due to their mechanical properties and biocompatibility, the uses of titanium dioxide coatings in biomedical sciences include drug delivery systems [59-61] and dental and orthopedic applications [62-65].
Since adequate interaction between the biomaterial and the surrounding tissue, as well as biodegradability, are desired properties of an orthopedic implant, magnesium alloys coated with titanium dioxide are of interest to biomedical sciences. By combining those properties, the lifetime of a magnesium implant can be extended and various biological processes can be fostered without waiving the biodegradation properties.
Titanium dioxide has been used as protective coating on titanium substrates, 316L stainless steel, and other metals [66], [67]. In spite of this, it is not easy to deposit TiO2 layers onto magnesium alloys due to the low adhesion and low homogeneity that some methods of surface modification provide [68]. Nevertheless, several authors have successfully managed to apply such coatings on magnesium and some of those studies are mentioned in the following paragraph.
In their work, Marin et al. [69] used the atomic layer deposition (ALD) technique for four different coatings. TiO2 / aluminum mono and multilayers were applied to the magnesium AZ31 substrate. The results showed that ALD deposition can be successfully used to protect the AZ31 Mg alloy from corrosion in aqueous solutions with low concentrations of NaCl. It is important to highlight that multilayer structures were the most effective against corrosion but long deposition times are the main limitation to this technique.
Ceramic coatings containing titanium dioxide nanoparticles and organic compounds -such as alginate- were placed on the magnesium AZ91D alloy by electrophoretic deposition (EPD), thus improving the corrosion resistance from 3 to 7 times when compared to uncoated magnesium. The results were obtained by electrochemical impedance spectroscopy (EIS) [70]. Hu et al. [68] produced titanium dioxide coatings onto AZ31 alloys by modifying the surface, using liquid phase deposition (LPD) followed by an annealing process. The TiO2 coatings -with or without annealing treatment- decreased the magnesium alloy degradation rate, compared to uncoated magnesium in electrochemical tests. Fujita and coworkers [71] researched the corrosion resistance of TiO2 commercial films on Mg by liquid phase deposition (LPD) and they found that by changing the solution’s pH -from acidic to highly alkaline along with the addition of sucrose- it becomes possible to form a highly adhesive and thin TiO2 film on pure magnesium without any heat treatment.
Recently Chen et al. [18] produced layers of polydopamine (PDA) between the TiO2 coatings and the Mg substrate to improve magnesium corrosion resistance. Covalently-immobilized PDA layers on Mg and TiO2 were obtained by deposition in liquid phase. The hybrid coating exhibited a significantly lower corrosion current, as well as a very low rate of in-vitro degradation (up to 21 days) in phosphate-buffered saline (PBS), compared to plain TiO2 coatings.
Meanwhile, Li et al. [72] studied the mechanical degradation of Mg-Ti composites in Hanks solution. For that purpose, they used titanium wires embedded in the magnesium matrix, employing the infiltration casting technique. The authors concluded that magnesium in the composite degrades more rapidly than pure magnesium. However, the strength of the composite was maintained at about 86 MPa after the Mg dissolved. Conversely, Bakhsheshi-Rad et al. [73] synthesized a nanostructured coating with hardystonite (HT) and titania/hardystonite onto Mg alloys. The results indicate that the degradation resistance of the magnesium alloys was improved, compared to an uncoated magnesium sample. It is emphasized that the cell viability was not affected by the exposure of the Mg coating with titania/hardystonite to preosteoblastic cells (MCT3-E1). Thus, the authors proposed new techniques to obtain coatings on the substrate, in order to control the corrosion rate. Similar data have been reported by Abdal-hay and coworkers [74]: titanium dioxide / poly (lactic acid) coatings on magnesium were used for biomedical applications, and they also showed a significant reduction of the biodegradation rate and hydrogen evolution of the magnesium substrate. These coatings were fabricated by electron beam physical vapor deposition (EB-PVD).
Micro arc oxidation has also been used to obtain porous surfaces of magnesium, followed by a TiO2 coating for sealing pores through the sol-gel method [75]. The authors conclude that the resistance to biodegradation in Hanks solution increases significantly -approximately 30 times- compared to uncoated magnesium only. The parameters of corrosion resistance were determined by electrochemical impedance spectroscopy and polarization assay after 12 hours of immersion in the solution. The authors also demonstrated that the coating provided a more stable surface over time.
Similarly, the study of coatings with nano silica-titanium dioxide on a Mg-Ca alloy by means of physical vapor deposition (PVD) has been reported. Coatings based on nano silica only were also evaluated. Electrochemical studies in simulated body fluid (SBF) showed that the nano Si / TiO2 surface presents a significant reduction in corrosion rates (0.57 mm / year), when compared to the sample coated with nano silica only (0.91 mm / year), or the uncoated sample (6.21 mm / year). Likewise, a low hydrogen evolution was also observed [76].
3.2. Sol-gel
The sol-gel method is an efficient alternative to obtain coatings on different materials, since it provides low-cost coatings with good adhesion [77]. The method also enables to control the structure of the materials at the nanoscale and it can be used to produce metal oxides.
Such method starts with the formation of a colloidal suspension of a molecular precursor in a solvent (sol) and a subsequent oxide network formation produced at low temperatures. The process involves two phases, signaled by the hydrolysis and condensation of the suspension and a polycondensation reaction that results in a three-dimensional network. The solvent is then removed from the gel by a drying process at room temperature (aging). Then, a heat treatment is applied to obtain monoliths or thin films. The sol-gel transition depends on the amount of precursors, water, catalyst, temperature and pH [78].
Several authors have used sol-gel methods for synthesizing TiO2 coatings. For example, Wang et al. [79] proposed a new method of dip/spin coating by sol-gel to prepare ultrafine titanium oxide films on α-Al2O3 disks. The method was different from others since the substrate was first immersed in the sol and it was subsequently removed to form a thin coating by spinning. The authors obtained small glass films with uniform grains and a unique structure. Advincula et al. [80] employed a sol-gel process to prepare titanium oxide ultrafine silicon wafers. Those coatings were later modified by self-assembled monolayers (SAM) of silanes with different functional groups, in order to evaluate their in-vitro protein adsorption behavior. The sol-gel process presents the advantage of accurately controlling the nanostructure and the thickness of these thin coatings. The authors suggested that surface modification with TiO2 by sol-gel and SAMs can promote protein adsorption in vitro, due to changes in the surface chemical composition, roughness, and wettability. Subsequently, Advincula et al. [81] evaluated the biological response of the titanium oxide matrix derived from sol-gel, reporting an increase in cell adhesion and matrix mineralization on the substrates. The adequate cellular response of titanium oxide by sol-gel has been described by other authors [82,83]. In addition, the literature has also reported [22,84] that sol-gel-prepared titanium dioxide is able to induce calcium phosphates formation on TiO2 surfaces, improving the adhesion of the material to the biological tissue.
3.2.1. Surface modification of magnesium with titanium dioxide by sol-gel
Titanium oxide coatings obtained by sol-gel have shown promising results. Recently, Li et al. [85] evaluated the corrosion resistance of coatings based on HA/TiO2 / PLA. Titanium dioxide was used as an intermediate layer and it provided high adhesion between the hydroxyapatite and the poly (lactic acid). The surface modification was obtained by the sol-gel technique through the dip-coating method, and the corrosion resistance of the studied surface improved when compared to uncoated substrates.
Different authors have evaluated surface modification of magnesium with titanium dioxide by the sol-gel technique. One of the studies was carried out by Ochsenbein et al. [83]. They analyzed the response of osteoblasts to different oxide coatings obtained by sol-gel, and the results showed that titanium dioxide fostered proliferation and cell adhesion after three days of incubation. Another similar study performed by Hu et al. [86] showed an anticorrosive effect of TiO2 coatings obtained by sol-gel on an AZ31 magnesium alloy with potential use in strong acidic environments. Furthermore, Hu et al. [87] assessed the in-vitro degradation of magnesium coated with a nano TiO2 film by sol-gel. They concluded that the corrosion current decreased by almost 3 orders of magnitude when compared to uncoated samples.
Li et al. [88] also showed that TiO2 coatings obtained by the sol-gel method significantly improve the corrosion resistance of a Mg-Ca alloy in SBF. More recent studies evaluated titanium oxide and titanium oxide / calcium phosphate coatings on AZ31 by the sol-gel method. Tang et al. [89] observed that the coatings improved the corrosion resistance and reduced the amount of hydrogen produced by magnesium corrosion. Although hydrogen bubbles are naturally created during degradation processes of magnesium alloys, the amount of hydrogen produced can decrease when the coating acts as a protection barrier. Corrosion studies were carried out in the solution of simulated body fluid (SBF). Moreover, Hernández et al [90] synthesized hybrid coatings by a mixture of organic precursors, inorganic glycidoxypropyltriethoxysilane (GPTMS), 3-aminopropyltriethoxysilane (APTES), and tetraethyl orthosilicate (TEOS) by the sol-gel route, in order to increase the corrosion resistance of the WE54-AE magnesium alloy. Generally speaking, the results showed a significant improvement in the corrosion current by about one order of magnitude.
In biomedical engineering, the improvement obtained by coatings on magnesium alloys can also be observed in the increase of cellular activity. The literature reports few studies on the biological properties of TiO2 substrates on magnesium. Still, some reports are available. Amaravathy et al. [91] successfully obtained sol-gel TiO2 coatings on a magnesium alloy -AZ31- and the results showed a significant improvement in adhesion during biological assessments. In addition, coated Mg exhibited a higher cell interaction (interaction area with the tissue) when compared to the unmodified alloy. Similarly, Amaravathy el al. [92] evaluated the coatings of titanium dioxide / hydroxyapatite on the magnesium alloy AZ31. The results of hydrogen evolution and potentiodynamic polarization studies showed that the coatings can act as a protective layer, decelerating the entry of corrosive metal ions from the SBF solution. Furthermore, cell cultures revealed that biocompatibility and cell adhesion of Mg coated with HA / TiO2 -evaluated by the osteoblast cell line MG63- improved significantly, which makes it a promising coating to induce osteointegration properties. TiO2 coatings on magnesium alloys usually create porous surfaces that may be unfavorable for applications of magnesium as orthopedic biomaterial. Surface defects may favor the rapid degradation of magnesium alloys. This problem has been solved by generating TiO2 multilayers on the Mg alloys. It has been described that a chemically alternated multilayer configuration generates greater resistance to corrosion [69]. Besides, it has also been reported that a correct densification of the TiO2 film prevents the formation of surfaces with pores or cracks [87]. New strategies include the use of bilayer-coatings and hybrid multilayer coatings using TiO2 and alumina [69] and TiO2 and hydroxyapatite [92] as coating materials.
4. Conclusions
Surface modification techniques are an important area in biomaterials research and biomedical engineering of magnesium alloys. By surface modification of magnesium and its alloys the biocompatibility can be enhanced and problems related to degradation of magnesium can be overcome while maintaining the bulk properties. Therefore, this paper reviewed TiO2 coatings used to improve the biological and corrosion resistance of magnesium and its alloys.
Research carried out during the previous 5 years has shown that the corrosion resistance and biological properties in the tissue-biomaterial interface of magnesium and its alloys may be selectively enhanced using appropriate surface treatments. Coatings obtained by sol-gel methods have proven to be attractive due to their low processing costs and because they enable to obtain homogeneous surfaces to decrease the degradation rate of the implant. Some studies have also reported that this technique can be used to create antibacterial surfaces, if suitable precursors are used.
Although there are multiple surface modification techniques to generate coatings on magnesium, considerable research efforts are still needed to meet the current clinical needs of biodegradable medical implants. Currently, there are no coatings on magnesium capable of maintaining mechanical integrity for the time needed by the bone tissue to heal and subsequently be successfully degraded. More efforts are needed to understand the interaction of magnesium coated with TiO2 with biological systems (mainly with cell lines derived from bone). There also are few studies available in the literature with results of the performance of in-vivo animal models. Likewise, studies showing antibacterial properties of coatings on magnesium are still under development.
Overall developments of new biodegradable magnesium alloys for medical use are based on the control of the corrosion rate and, thereby, the mechanical integrity of the material in a physiological environment. Several research papers published during the previous 5 years showed that coatings with titanium dioxide significantly improve the surface properties of magnesium. Surface treatments with new and innovative materials can reduce the corrosion rate and favor integration between the bone and the implant.