1. Introduction
Industrial activities can generate significant loads of pollutants that are released into water sources. Gold mining uses highly toxic compounds such as cyanide [1] which is considered potentially lethal to any ecosystem. In contact with water it produces hydrocyanic acid, a compound that causes serious illnesses in humans and animals, and degradation of soil fertility. The use of cyanide in gold mining is an important cause of biodiversity depletion in developing countries such as Colombia [2].
Water quality studies have been carried out in mining areas in Colombia (i.e. Marmato - Caldas) and have reported concentrations of cyanide ion as high as 5.60 ppm [3], amounts that exceed the Threshold Limit Value - Short-term Exposure Limit (TLV - STEL) established at 4.7 ppm [4]. Current methods to remove cyanide in aqueous medium are generally expensive and not very efficient [5] since they involve modifications in the extraction process and wastewater management that implies the incorporation of equipment and reagents [6]. A practical alternative is the adsorption and removal of cyanide from aqueous media through the use of lignocellulosic materials; Luffa Cylindrica is made up of cellulose and lignin, structures that have functional groups and provide the possibility of forming bonds that favor adsorption also, natural sponge forms a fibrous network with macropores of a diameter between 10 and 20 μm, feature that, also influences the process [7]. In Colombia the Luffa Cylindrica sponge is a feasible choice to be used in cyanide removal because of its availability and low cost. On the other hand, the natural sponge can be used as a support for microorganisms that degrade cyanide [8,9]. The main objective of this study was to evaluate the natural sponge Luffa Cylindrica as an adsorbent material to retain naturally occurring cyanide in an aqueous medium. In order to obtain an efficient model, an experimental design was carried out considering the main experimental variables.
2. Materials and methods
2.1. Material pretreatment
The Luffa Cylindrica samples come from the department of Valle del Cauca - Colombia. In order to increase the material contact surface its particle size is reduced, then the raw material is washed with distilled water and dried at 80°C which removes possible interference that could influence the adsorption process and finally, the sample is homogenized at 850 µm.
2.2. Material characterization
An acid-base titration with standardized solutions of HCl and NaOH 0.01N, using 0.5000 g adsorbent material and point of zero charge through NaCl electrolytic medium. Sample solution amounts between 0.1000 - 0.6000g [10].
Spectrophotometry FTIR: an IR-Prestige Shimadzu spectrometer was used to obtain infrared spectra under ambient conditions.
Stereoscopic Images: samples of the wet material were observed in a binocular Leica stereoscope with 100x magnification.
BET area: the determination of the respective nitrogen isotherm at 77 K is performed in Quantachrome Autosorb Automated equipment.
2.3. Adsorption tests
The cyanide ion in solution was quantified by ultraviolet spectrophotometry through the formation of cyanogen chloride. Reaction with pyridine to give glutacondialdehyde in condensation with 1,3-dimethylbarbituric acid, resulting in a violet colored complex with polimetine maximum absorption at a wavelength of 585 nm.
2.4. Experimental design
The appropriate adsorption conditions of cyanide by the natural sponge were determined through a factorial experimental design 22 where we evaluated two variables in two levels: contact time (10 and 60 min) and pH (9,0 and 13) as shown in Table 1. The experiments in the research were the result of duplicates studied by analysis of variance and a Tukey test with Statgraphics plus 5.1® program. Differences were considered statistically significant when the p value was <0.05.
2.5. Adsorption isotherms
Adsorption tests were performed at 20 °C, using 0.500 g adsorbent materials and concentrations of cyanide solutions in a range of 15 - 50 ppm.
3. Results and discussion
3.1. Characterization of the adsorbent material
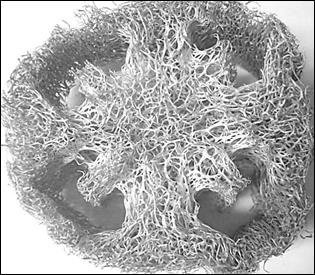
Luffa Cylindrica natural sponge is composed of 60% cellulose, hemicellulose 30% and lignin 20% [13], a fact that makes it a flexible and durable material. This composition reflects not only its physical-chemical characteristics but also in the material. Fig. 1a shows a porous structure composed of a thin outer layer of fibers that connect inside forming a complex network of fibers. In the stereoscopic image in Fig. 1b it can be seen that fibrils are oriented parallel to the longitudinal axis of each fiber [14].
3.1.1. Superficial chemical
Table 2 shows the quantification of acid and basic sites, which allows us to establish the superficial chemical characteristics of the material; a value of 0.84 meq/g indicates greater concentration of groups of acid character, and suggests a predominance of groups type hydroxyl and carboxyl, consisting mainly of lignocellulose that can actively intervene in the process of adsorption material.
The zero charge point makes reference to the total surface load and it establishes a value of 4.5 for natural sponge; it shows a positive superficial charge to solutions of lower than 4.5 pH and negative to solutions greater than this value. [10].
A relevant parameter to take into account about the material that is used as an adsorbent is the specific superficial area (BET); the considered value, turns out to be too small but coherent, since it is within the rank that is established under study for this kind of material, and whose value approximates to 1.04 m2/g [15].
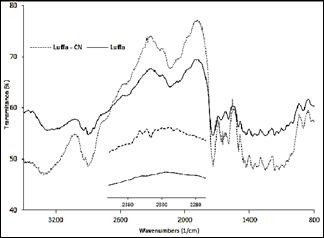
The chemical structure of the absorbent material is relevant to understand the adsorption process. Identification of functional groups on the surface was carried out through the respective spectra FTIR which is in Fig. 2.
In the spectrum that corresponds to the natural sponge Luffa Cylindrica it is observed that a band at 899 cm-1 is attributed to an extension of the bond (C(C [16]. The signals that are located between 2856 and 2925 cm-1 are due to the symmetrical absorption in the bond (C(H [17]. The band located at 1736 cm-1 is due to C(O bonds, which is characterized by its acute formation and relative isolation from other bands [18]. Also, the peak at 1672 cm-1 is assigned to the presence of (NH2. The peaks ranging between 1300 and 1100 cm-1 may correspond to ketones.
The obvious difference between both spectra show the interaction between the cyanide ion and the material that is used as adsorbent, the appearance of the band in 2343 cm-1 is related to the presence of carbonyl groups, which can react with adsorbate once it is retained in the surface of the material [19].
3.2. Experimental design
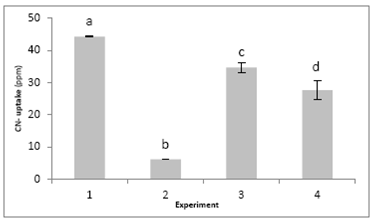
The results obtained when evaluating cyanide ion retention in the natural sponge Luffa Cylindrica applying the experimental design proposed is shown in Fig. 3.
The ANOVA of factors applied to the factorial design indicated that the pH effect (p=0.00007), contact time (p=0.00689) and the interaction pH-contact time (p=0.00029) are statistically significant (p<0.05) in the retention of the cyanide ion in Luffa Cylindrica. Therefore, the results of the analyses of variance between the interactions are illustrated in Fig. 3. These indicate that the number one experiment in which the conditions correspond to contact time 10 minutes and pH 9.0 allowed to obtain the highest percentage of retention of cyanide ion. It is also possible to establish that there are statistically significant differences (p<0.05), between the experiment 1 and the other evaluated systems. Therefore, this experiment was found to be the most suitable for adsorption of ion cyanide by natural sponge.
Source: The authors
Figure 3 Cyanide ion concentration retained in natural sponge according to the experimental design. Letters indicate statistical differences (P=0.05). The values in each experiment are the average of two determinations using standard deviation to calculate the deviation.
3.3. Adsorption isotherm
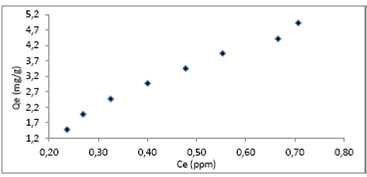
Characteristics of the adsorption process of a cyanide-Luffa Cylindrica system can be observed in Fig 4; trend data indicate a typical adsorption isotherm on heterogeneous surfaces, which turn out to be representative of the Freundlich model.
It is observed that the curve continuously increases, which could indicate that it has not reached the saturation point of the material and that in principle the initial adsorbate concentrations may exceed the maximum established in mining.
Considering that in the cyanide solution pH value at a value of 9.0 the majority of adsorption behavior could be attributed to the presence of acidic basic functional groups and in the particular case of the Luffa Cylindrica to its porous structure.
The evaluation process of the cyanide ion adsorption in aqueous solution of lignocellulose material was carried out by using the Langmuir and Freundlich models; the corresponding linearization parameters are presented in Table 3.
The linear correlation of coefficients allows us to define the surface on which the adsorption process is carried out on the closest or equal values to the unit. Represented specifically by the Freundlich model, they establish heterogeneity of surface in the adsorbent that is used. The constant KF in this model is associated with the adsorption capacity [20], for the system under study at 7.08 mg(g-1. One important parameter is NF, since it provides favorable conditions for the adsorption process when it is over 1.0, a fact that is demonstrated in the evaluated material.
3.4. Adsorption kinetics
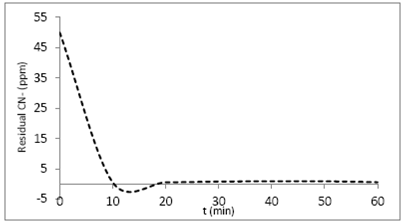
The kinetic behavior of the system is shown in Fig. 5; the adsorption process takes place rapidly during the first 10 minutes, after an apparent steady state occurs. It is noteworthy that between 10 and 20 minutes the graph takes the form of a potential well, which could be attributed to the material surface, composed by a diverse array of pores that are very close to each other [21].
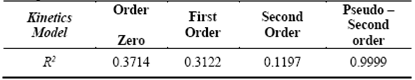
The natural sponge-cyanide ion system was evaluated under four different conventional kinetic models, which are summarized in Table 4.
The results show correlated values (R2), highly significant in the systems represented by the pseudo second order model, which could indicate that the adsorption system involves strong interactions via hydrogen bonds [22]. The values corresponding to the rate constant (K2), as well as the initial rate of adsorption (h), are shown in Table 5.
The initial rate proved to be slightly high, a fact that might suggest multiple interactions between the cyanide ion and the lignocellulose adsorbent material [23].
3.5. Real matrix
The result of a single test represented a cyanide ion retention in aqueous media of 98.2 %, which means quite high values in the real matrix, as this simulates water conditions resulting from the process of gold mining, where the presence of ions such as zinc and copper [24], may have effects in the adsorption process of the cyanide ion.
4. Conclusions
The suitable conditions to carry out the process of adsorption of the cyanide ion on natural sponge Luffa Cylindrica are pH 9.0 and an equilibrium time of 10 minutes, likewise, the system is mainly represented by the Freundlich model, which involves adsorption on heterogeneous surfaces. The kinetics adsorption of the system under study follows the pseudo- second model order. The lignocellulose material tested in the real matrix has a high retention efficiency of the ion of interest, as retention values approach 98.2 %, indicating an excellent option for treatment of water that has been contaminated with cyanide by the gold mining process.