1. Introduction
Most microorganisms considered as probiotics belong to the lactic acid bacteria (LAB) where the most representative genera of sporulated bacilli includes Lactobacillus sp., Lactococcus sp., Bifidobacterium sp., and Bacillus sp. These bacteria produce a series of metabolites such as lactic acid, hydrogen peroxide, aromatic compounds (diacetyl, acetaldehyde), and other bacteriocins that are capable of inhibiting the growth of pathogenic organisms therein generating cell lysis [1]. There are several studies on the beneficial properties attributed to the probiotic microorganisms to human health, that describes how these bacteria act as a barrier protecting the gastrointestinal tract, inducing activation of the immune system and contributing to better digestion and absorption of nutrients [2]. For all these reasons, probiotics have been considered of great biotechnological value, being widely used in controlling pathogens, to prevent both bacterial and fungal infections in animals and in man [3].
One of the challenges for food technology regarding the addition of probiotics in food, is to ensure that these microorganisms arrive alive and in good condition to their action site within the body of the host, and ensure implementation in a wider range food. The survival of these bacteria, depends on the vehicle in which they were built, therefore, it is necessary to generate strategies to ensure their survival in the matrix where they are incorporated, during storage and their passage through the gastrointestinal tract [4].
Among the technologies proposed to ensure cell viability of these microorganisms in food, microencapsulation technique offers protection to probiotic microorganisms against adverse conditions. Several studies are related to this subject such as a research carried out by Khosravi Zanjani, Tarzi, Sharifan, & Mohammadi (2014), who aimed to study the protective role of this technique in the bacterias Lactobacillus casei and Bifidobacterium bifidum, via emulsion technique. Mokarram, Mortazavi, Najafi, & Shahidi, (2009), demonstrated that microencapsulation of Lactobacillus acidophilus and Lactobacillus rhamnosus, ensures its survival against temperature and pH changes. Also in the study of Ding & Shah, (2009), the spray-drying technology was the most often used in the food industry for probiotics microencapsulation [5-7].
According to the above, in the present study, cell viability of three types of probiotic bacteria was evaluated: Bacillus polymyxa, Bacillus megaterium and Lactobacillus delbruekii ssp. bulgaricus, microencapasulated under spray drying technique with maltodextrin more inulin, as well as the effect of pH, bile salts and temperature on the microcapsules obtained.
2. Materials and methods
2.1. Activation of strains
Probiotic bacteria (B. polymyxa, B. megaterium and L. delbruekii ssp bulgaricus) were granted by Corporación Universitaria Lasallista and previously isolated, characterized, and stored at 80 °C in glycerol 7.5%. B. polymyxa, and B. megaterium activation was produced by sowing the strains with the exhaustion agar Plate Count technique (Merk KGaA, Darmstadt, Germany) at 37 ºC for 48 hours under aerobic conditions and L. delbruekii ssp bulgaricus in M17 agar (Merck KgaA, Darmstadt, Germany) at 37 ° C for 48 hours under anaerobic conditions.
After incubation period, colonies were recovered separately in a sterile solution of deionized water to reach a final count of 3x109 bacteria million cells / mL according to the Mc. Farland 10 pattern. Volumes obtained were mixed in equal proportions, and to this solution was added the corresponding concentration of maltodextrin and inulin (Tecnas, Itagüí, Colombia).
2.2. Microencapsulation process
BÜCHI Mini Spray Dryer B-290 Swiss-made was used for microencapsulation. Optimization of microencapsulation processes was obtained through a central composite design with five replicates in the center with the help of Statgraphics. The response variable, survival of microencapsulated bacteria was evaluated, measuring the number of colony forming units per gram (CFU / g).
2.3. Spray drying technique using maltodextrin and inulin
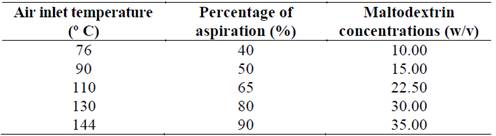
Suspensions with maltodextrin and inulin were prepared following the protocol described by Rodriguez, (2012). Maltodextrin concentrations used here were 10 to 35% w / v in a suspension with a prebiotic and cells in a ratio of 1:1 (w/v) with a final concentration of microorganisms 3x109 CFU / mL. This preparation was homogenized at 4000 rpm for 10 minutes and maintained under constant stirring at room temperature during the power supply, where an air inlet temperature range between 76-144 °C, a percentage of aspiration between 40 % and 90 %, an airflow of 450-565 L/h and a feed flow of 6 mL /minute was used (Table 1). Microencapsulated material was collected and sealed in sterilized foil pouches and stored at room temperature and at 4 °C.
2.4. Response surface methodology
To estimate the optimal levels of the variables needed for the encapsulation process that would ensure greater viability, a central composite design was applied with three independent variables: temperature input (X1), percentage of aspiration (X2) and maltodextrin concentration (X3). The dependent variable: viability of probiotic bacteria (Y). The number of experiments was calculated based on the following equation: 1) 2K + 2 (K) + Cp = 23 + 2 (3) +5 = 19; where K = number of independent variables, and Cp = central point of the design.
2.5. Evaluation of survival microencapsulated probiotic bacteria
In order to determine the survival of microencapsulated probiotic bacteria, counting was assessed by CFU subjecting the microcapsules to serial dilutions in PBS (phosphate buffered saline), then seeded in selective culture media for each bacterial strain and survival was determined by counting colony forming units per gram (CFU / g).
The temperature, rate of aspiration and maltodextrin concentration that obtained the highest bacterial count in units’ formers Cologne per gram (CFU/g) was selected, and the effect of pH 2.5 and bile salt concentration was evaluated at 0.3 % p/v for 4 hours. Similarly, stability of the microcapsules to temperature was determined and subjected to 10 minutes of heating at 70 °C, then viability was evaluated. Likewise, they were stored for 1, 30, and 60 days at temperatures of 4 °C and 25 °C in order to assess its strength and viability over time. All experiments were done in triplicate.
The colonies recovered were subjected to Gram staining and biochemical tests API® 50 CHL and CHB bioMerieux to confirm that it was initially used strains and discard any contamination.
3. Results and discussion
The recovery percentage of microencapsulated material in grams was averaged in 40 % per 100 mL of solution.
When assessing the effect of spray drying process on the viability of the three probiotic strains contained in the suspensions with maltodextrin more inulin, growth in both, sporulated bacilli and lactobacilli was evidenced. Gram staining and biochemical tests of recovered colonies confirmed that were the baseline strains. These results confirm other studies where the survival of encapsulated probiotic based on this method was significantly higher compared to the free microorganisms, and ensure the stability of such microorganisms in functional food products [1].
Other studies have also reported the effectiveness of this technique in the microencapsulation of probiotic, including lactic acid bacteria and sporulated bacilli [8-10].
According to response surface analysis the most important effect for the survival of microorganisms was the temperature (X1) (Fig. 1).
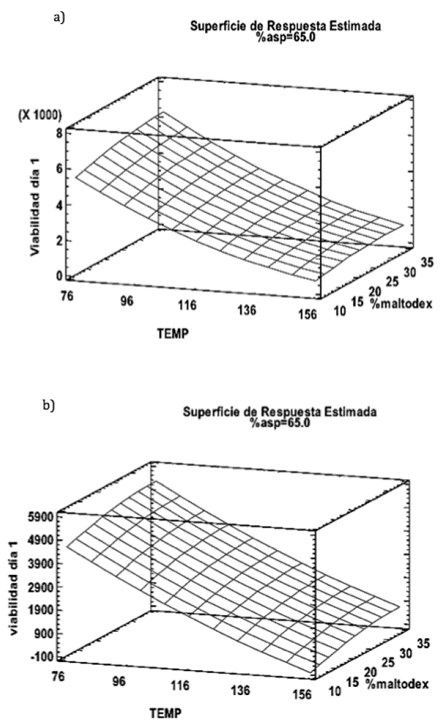
Source: The authors.
Figure 1 a) Estimated Response surface for the survival of sporulated bacilli. b) estimated response surface for the survival of lactic acid bacteria.
Most viable microorganisms were recovered at 76 °C where the survival average for sporulated bacilli at day 1 was 6,33x106 CFU / g and for lactic acid bacteria was 5,12x106 CFU/gr. However, when the viability of such microorganisms was evaluated at 144 °C a count of 1.28 x 106 CFU / g for sporulated bacilli and 1.08 x 106 CFU / g for lactic acid bacteria was obtained. These data indicate that the greater tolerance by sporulated bacilli against high temperatures is probably due to the ability of these microorganisms to form spores, which allows them to tolerate extreme environmental conditions. Likewise, previous studies have claimed that the characteristics of each strain and tolerance to different stress levels are crucial for their viability when subjected to these procedures [11].
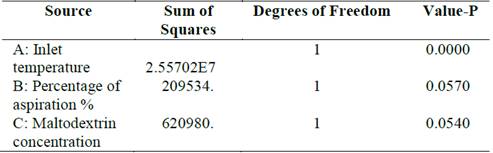
Moreover, analysis of variance for the viability of the sporulated bacilli indicated that there were statistically significant differences in the interaction between the viability of microorganisms and temperature (p <0.05), while the concentration of maltodextrin and the percentage of aspiration showed have no a significant effect (Table 2).
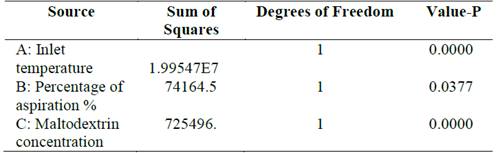
Lactic bacteria however, showed a significant difference (p <0.05) for the CFU counts with respect to temperature, the rate of aspiration and concentration of maltodextrin (Table 3). Similarly, analysis of variance for viability carried out between these groups of bacteria showed a significant difference (p <0.05).
According to other studies, the viability of lactic acid bacteria depends greatly on the drying temperatures to be used during this process, due to damage that can occur on the cell membrane, also parameters such as air flow and encapsulant material also affect the survival of these bacteria [12]. During storage of the microcapsules, a continuous decrease in the viability of both groups of microorganisms was observed. There was no significant difference for the microencapsulated bacteria count when stored at 25 ° C compared to 4 °C and viability, both, day 30 and day 60 was obtained. The average count of sporulated bacilli obtained for day 30 was 3.11x106 CFU / g and for day 60 2.84 x106 CFU/g, while for lactic acid bacteria an average of 2.44 x 106 CFU/g was obtained for day 30 and 1.70 x 106 CFU/g for day 60, with an increase in the count of CFU/g of the encapsulated material stored at 4 ° C with respect to the stored at 25 ° C. Studies reported so and obtained a greater reduction in the number of microorganisms encapsulated stored at 25 °C because at this temperature the metabolic activity is higher and nutrients are consumed quickly [13]. Similarly, other studied have shown that microencapsulation with maltodextrin and thermoprotectant agents like inulin increase the microorganisms survival after drying and during storage over 21 days at room temperature, because is minimized mechanical, oxidative and osmotic stress to which they are subjected [14].
Variables values that optimized microorganism’s viability in the microcapsules were: temperature 76 °C, percentage of aspiration of 60 % and maltodextrin concentration of 35 % w/v, with a count of 6.7x106 CFU / g for Bacillus and 5.4X106 CFU / g for Lactobacillus (Fig. 2).
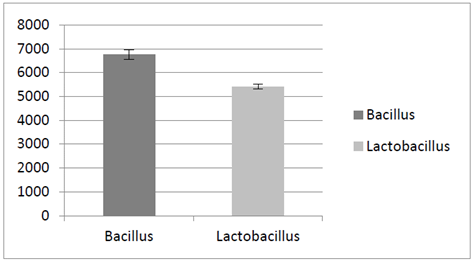
Source: The autors.
Figure 2 CFU Counts for bacilli and lactic acid bacteria under variables that optimized viability in the microcapsules: 76 °C, percentage of aspiration of 60 % and maltodextrin concentration of 35 %.
These results are consistent with investigations where the authors used air inlet temperature ranges between 70 and 100 for achieving a microbial survival lactic bacteria of 60 % [15]. We also found a significant difference (p <0.05) for the strains under the effects of temperature, pH and concentration of bile salts with 3.7 x 106 CFU / g regarding to the strains unexposed with 6 x 106 CFU/g ( Fig. 3).
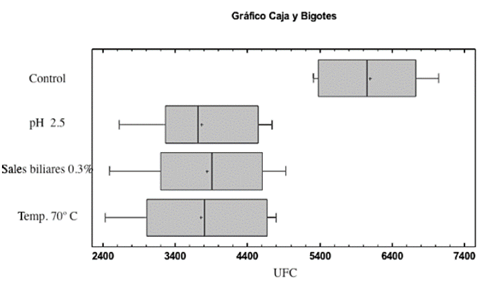
Source: The autors.
Figure 3 Comparison of the strains subjected to the effects of pH 2.5, 0.3 % bile salts and 70 °C temperature versus control strains.
In the same way, other studies showed survival in the microcapsules of Lactobacillus acidophilus after being subjected to pH conditions of 2.0 and 0.5 % bile salts and 1 % for six hours of incubation [16].
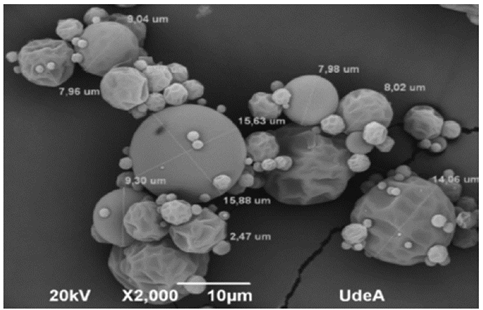
The electronic photomicrography showed circular and irregular particles with sizes ranging from 7 to 15 micrometres on average (Fig. 4). Similar data were obtained in other studies with microencapsulated material consisting of spherical particles with shape and sizes of 5.21 ± 4,16 μm on average and typical concavities produced by spray drying due to shrinkage that occurs during the process [14].
4. Conclusions
Microencapsulation under the spray drying technique is an alternative to maintain the integrity of the probiotic strains facilitating their incorporation in various food and pharmaceutical matrices. Also protects the bacteria from temperatures of 70 °C and retain their stability after being subjected to conditions simulating the digestive system such as pH 2.5 and bile salt concentration of 0.3 % w/v, ensuring the minimum concentration of 1x106 CFU / gr, recommended by the Food and Drug Administration (FDA), Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO).