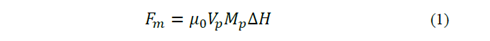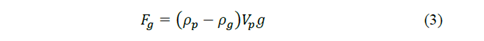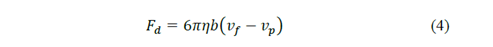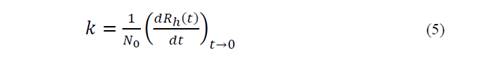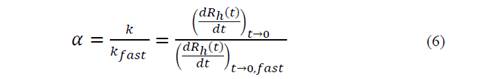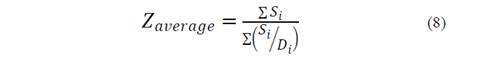1. Introduction
The large-scale application of nanomaterials in the field of remediation has been limited by different factors ranging from economic to difficulties in implementation and scaling. One of these factors - in the context of using and reusing management - is the separation of the nanomaterials from the aqueous medium after performing their function, since conventional techniques such as precipitation, centrifugation or filtration are in some cases inefficient or costly [1-3].
For this reason, the implementation of magnetic nanoparticles (MNPs) has been seen as one of the most promising alternatives, since apart from its potential as a remediation agent is added the fact that their separation of the aqueous medium is simpler and more efficient [4-6].
The process of separating MNPs from the aqueous solution is based on cooperative phenomena of magnetophoresis, is a reorientation of the magnetic moment that holds particles under the action of non-uniform external magnetic fields towards the highest field gradient. The magnetic force involved can be expressed as [7]:
where μ 0 is the permeability constant in vacuum, 𝑉 𝑝 is the particle volume, 𝑀 𝑝 is the magnetization of the particle and ΔH is the gradient of the magnetic field strength at the particle position. Also, the magnetization of the particle is given as a function of the volumetric magnetic susceptibility (𝜒) and the field strength applied [8]:
Also, it is important consider the gravitational force 𝐹 𝑔 and the hydrodynamic drag force 𝐹 𝑑 [7,9,10]. For the gravitational force is fulfilled:
And for the drag force:
where 𝜌 𝑝 is the density of the particle, b is a constant that depends on the properties of the fluid and the dimensions of the particle, 𝜌 𝑔 the density of the fluid, 𝑔 the gravity, 𝜂 the viscosity of the fluid and 𝑣 𝑓 and 𝑣 𝑝 are the velocities of the fluid and the particle respectively.
As seen in the equations 3 and 4, the volume - and hence the size - of particles plays a fundamental role in the recovery process. However, the increase in particle size leads to a decrease in the reactivity and removal capacity of the contaminant as it reduces the surface area/volume ratio [11,12]. For this reason, the study of the dynamics of aggregation of particles in water takes relevance.
Nanoparticles with a high free surface energy tend to aggregate, in trend toward stability. The smaller particles have a greater aggregation tendency due to lower energy barriers [13,14]. Aggregate formation decreases the surface area of magnetic nanoparticles. This reduces the reactivity, thus limiting the performance in catching pollutants. Aggregation also reduces the effectiveness of magnetic nanoparticles during remediation due to loss of Brownian mobility [15].
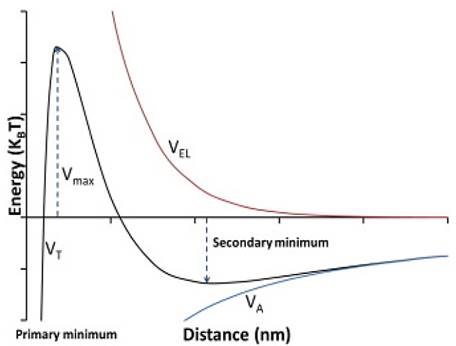
The most appropriate way to study this phenomenon is the DVLO model. The traditional DLVO (Fig. 1) theory describes colloidal stability by considering the total energy of the interaction in a particle with another particle. The main interaction energy can be estimated and calculated as the sum of Van der Waals and the double layer of electrical interactions [2,16,17].
2. Methodology
2.1 Materials
Iron (III) chloride hexahydrate (FeCl3∙6H2O) and Sodium hydroxide (NaOH) were purchased from Merck Colombia. Reduced L-Glutathione (C10H17N3O6S) was purchased from Sigma-Aldrich. All reactive were analytically pure and used as received without further purification, and all solutions were prepared with double-distilled water.
2.2 Samples preparation
The Fe3O4 NPs obtained through the green method (Glutathione@MNPs) were synthesized using an improved method described by [19] . Briefly, we added 10 mL of a FeCl3 (0,1 mol/L) solution to a 100-mL beaker under mechanical stirring (140 rpm) at 75°C. On reaching this temperature, 20 mL of an aqueous solution of L-Glutathione (0,214mol/L) were added dropwise into the beaker with the pH value of the mixed solution adjusted to 10. The mixture was brought to a temperature of 85°C and this solution was stirred (120 rpm) for another hour. At the end of this period, the nanoparticles were separated by magnetic field application and then washed several times with deionized water and alcohol, and then vacuum dried at 40°C for 12 hours.
2.3 Characterization
The particle size and morphology of all the synthesized nanoparticles were characterized using transmission electron microscopy (CARL ZEISS MODEL EVO HD MA 15). The magnetic properties of the nanoparticles were studied through vibrating sample magnetometer (VSM Lakeshore, Model 665) measurements. The particle size was measured through the SEM and dynamic light scattering (DLS) methods, the size distribution of the particles and Zeta Potential were determined using a Malvern Zetasizer Nano (ZS90).
2.4 Agglomeration kinetics studies
In order to establish the aggregation rates of Glutathione@MNPs, suspensions of this material were prepared in deionized water at a concentration of 20 mg / L and sonicated for 10 minutes at 250 W. Thereafter, temporal changes in hydrodynamic radius as a function of ionic strength were measured by Dynamic Light Scattering (DLS) using a Zetasizer Nano ZS instrument (Malvern Instruments, UK). It has a 633nm laser as the light source and the intensity of the light scattered by the particles is measured as the photon count rate by a detector at 173°. Particle size is determined by the analysis of the fluctuation of light intensity diffused by particles subjected to Brownian motion.
With the measurement of these changes in the hydrodynamic radius from an initial value R_h0 to 1,25Rh0, the initial rate of aggregation of the nanoparticles (k) is determined [20,21]:
where N is the concentration of the particles. With this value, it was possible to determine the fixation efficiency and easily obtained from the relation of the initial aggregation rate to a given chemical condition of the solution with the aggregation rate constant measured under conditions bound by diffusion (rapid) [20-22]:
In order to establish the concentration that decreases the potential barrier causing the flocculation of the nanoparticles and which ions normally found in surface water like rivers (Na1+, Zn1+, Ca2+), concentration is tabulated 5 to 30 mg/L) of electrolyte at the intersection of the reaction-limited stability curve (extrapolated from the linear section of the real stability curve) and the diffusion-limited stability curve (constant α value) and Thus finding the critical concentration of CCC coagulation [22-24].
2.5 Studies of magnetic sedimentation
Looking forward to studying the feasibility of the separation of the nanoparticles after being implemented in a treatment process, the study of the sedimentation of the nanomaterial was carried out as a function of time and the applied magnetic field. The magnetic system that was implemented produced a magnetic field gradient from a neodymium magnet cylinder with a diameter of 40mm and a height of 5mm that produced a field of 0,2 T. The suspension of nanoparticles in water was carried out in a volume of 250 ml where the influence of the magnetic field was measured in absence and presence of it by measuring the turbidity of the solution with a turbidimeter at different pH values (4, 7 and 9). Similarly, the influence of ionic strength (Na1+, Zn1+, Ca2+) at different concentrations (10, 20 and 30 mg/L) as a function of time. On the other hand, the sedimentation of the magnetic nanoparticles after the adsorption of heavy metals (Hg and Cr) was also evaluated as a function of time.
With this, the percentage separation of the magnetic nanoparticles from the aqueous phase over a period was calculated as follows:
3. Results and discussion
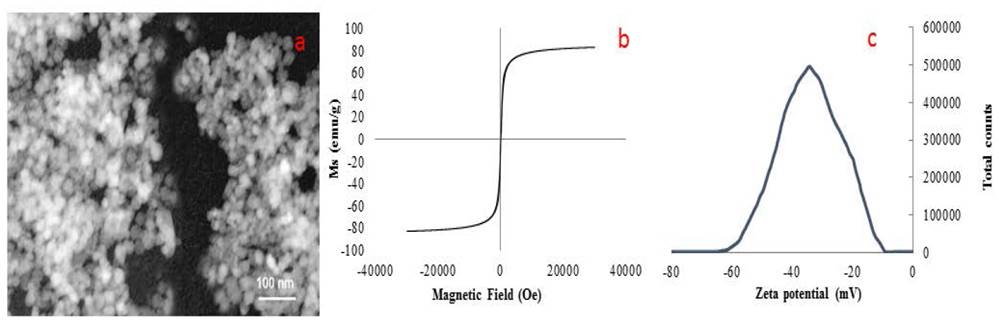
Characterization of Magnetic nanoparticles synthesized by the green methodology using glutathione with reducing agent and stabilizer can be observed in Fig. 2. TEM image (Fig. 2a) shows an average size of 60 nm. Saturation magnetization curve of the nanomaterial at room temperature (Fig. 2b.) was 85.4 emu/g, which is a close to theoretical value and a little larger to conventional synthesis which are 90 and 82.4 emu/g respectively. The surface charge of the material was determined by measuring the zeta potential (Fig. 2c.). A surface charge of -35.5 mV was obtained. This value represents the repulsion force of groups on the structure of glutathione (carbonyl and the very weak acid behavior of 1° and 2° amines), in order to stabilize the colloidal solution [25].
Source: The authors
Figure 2 Characterization of Glutathione@Fe3O4 NPs. a) TEM image, b) Room temperature magnetization and c) Zeta potential of Glutathione@MNPs.
3.1 Kinetic aggregation studies
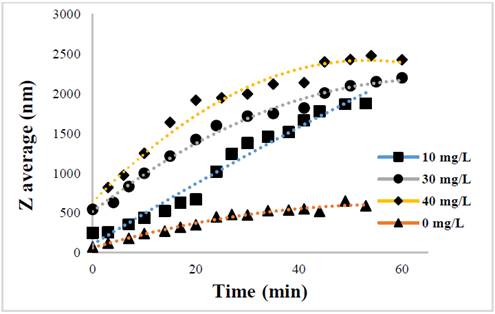
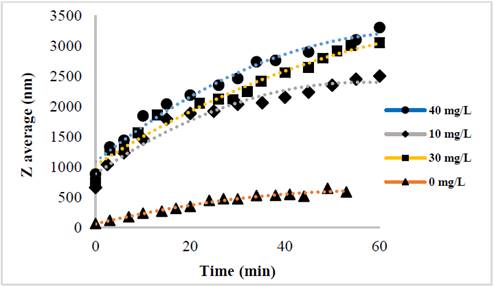
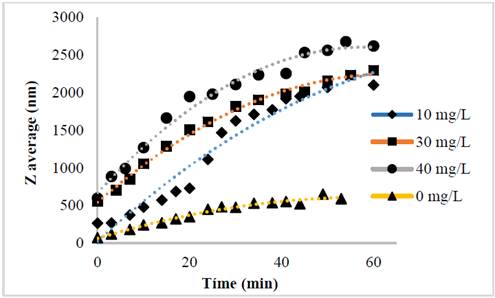
The evolution of the hydrodynamic radio profile of the magnetic nanoparticles modified with Glutathione over time in relation to the concentration of the ions in solution can be observed in Figs. 3 to 5. The Z-average can be expressed as the intensity based harmonic mean and is shown by the equation below [26]:
Here, S i is the scattered intensity from particle i and D i is the diameter of particle i. Note that the result is in the form of a harmonic mean.
Taking into account the behavior of the nanoparticles in these media it can be observed that the increase in the ionic strength in the solutions decreases the steric repulsion force between the particles given by the charges of the amino, carboxyl and thiol groups on the surface of the material. This is because interactions between the charges of the ions and the loads of these functional groups are presented [27-31]. The conditions under which the rapid initial aggregation occurs in a specific system may be related to its CCC, which is discussed below. Likewise, the agglomeration phenomenon in the solution that does not have ions in solution also takes place although they are low or despicable, processes of oxidation of the particle and electrostatic interactions between the positive and negative charges of the different functional groups occur [14,32-35].
This decrease in charges could be evaluated in the Zeta potential analysis, where the relationship between the surface charge of the particle and the concentration of the electrolytes in the aqueous solution can be seen in Fig. 6. Since the pH of the solutions after the addition of the nanoparticles was approximately 7, we can establish the decrease in the stability of the material since the long absolute value was higher under conditions of absence of electrolytes regarding to the addition of these.
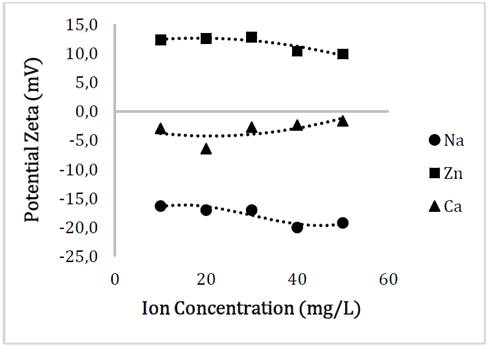
Source: The authors
Figure 6 Behavior of the surface charge of Glutathione@MNPs as a function of the concentration of Na, Zn and Ca ions.
The efficiency of binding or agglomeration of Glutathione@MNPs as a function of the concentration of the medium and the concentration of the electrolytes can be seen in Fig. 7.
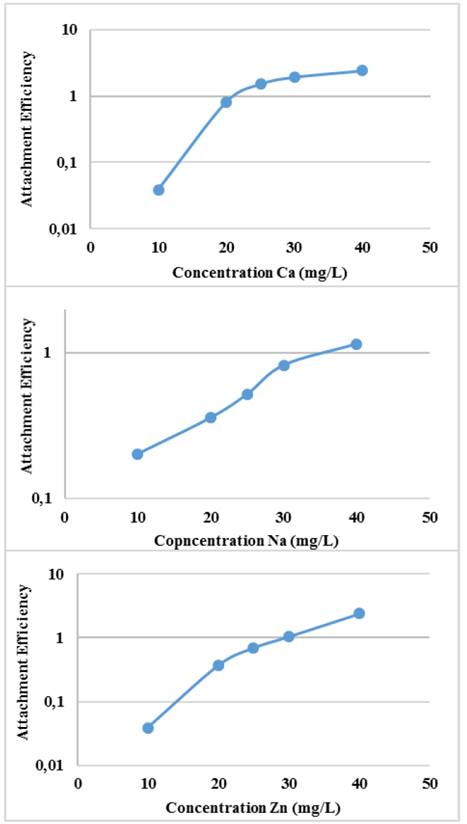
Source: The authors
Figure 7 Agglomeration efficiency of Glutathione@MNPs in aqueous systems with ion salts at different concentration.
The binding efficiency profile shows typical DLVO behavior with two distinct regions. The former is known as limited reaction agglomeration (RLA), where binding efficiency gradually increases with increased concentrations of media and electrolytes due to the decreased surface charge of the nanoparticles. The second is known as diffusion-limited agglomeration (DLA), where the binding efficiency reaches a constant value in certain media and known electrolyte concentrations (CCC). The CCC values found are presented in Table 1.
Table 1 Critical Coagulation Concentration of Glutathione@MNPs with different salts in aqueous medium.

Source: The Authors
Other reported values of critical coagulation concentrations have been reported for materials of similar chemical structure. In [36,37] a CCC value for Hematite nanoparticles of about 65Mm (1495 mg Na/L) was reported at near neutral pH when NaCl was used as the sodium precursor salt. Compared with the obtained value of 30 mg/L, the required amount of the salt would be lower to promote the precipitation and separation of the magnetic material from the aqueous solution.
3.2 Studies of magnetic sedimentation
Fig. 8 shows the sedimentation dynamics of Glutathione@MNPs in aqueous solution in the absence and presence of a magnetic field at different pH values. Suspensions of particles in the absence of magnetic field and affected only by the gravitational one remains stable for several weeks (approximately 8) which shows greater stability regarding to unmodified MNPs which normally show agglomeration and precipitation after 2 weeks. This could have important implications for the transport behavior of pollutants in the natural environment because, due to the high reactivity of the nanoparticles, the contaminants could be incorporated into the nanoparticles through adsorption, co-precipitation and other geochemical processes [37,38]. Therefore, stable suspended MNPs could improve transport of contaminants.
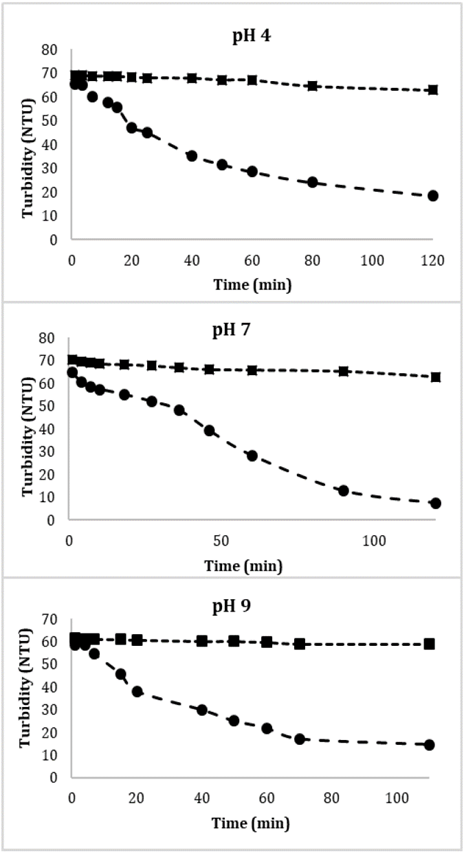
Source: The authors
Figure 8 Effect of the magnetic gradient on the sedimentation dynamics of Glutathione@MNPs in aqueous systems at different pH values. ●With magnetic field, ■ without magnetic field.
In contrast, the particles in the presence of the applied field suffer an acceleration in sedimentation velocity since in a period of 2 hours more than 80% of these are attracted by the magnetic field. The remaining 20% of the particles in that period have not yet undergone processes of agglomeration and/or attraction due to their size or the strength of the steric forces of the functional groups belonging to Glutathione, in addition, a steric interaction due to the formation of a layer of water around the material could also be important, however so far there is no adequate model to quantitatively estimate such contribution [39].
Aqueous suspensions of Glutathione@MNPs with small additions of salts in the absence of a magnetic field remain stable for a long time (2 weeks) but less time than in the absence of the salts. The difference in stability based on the nature of salt was expressed in greater stability with Zn > Na > Ca. Despite this difference, when the nanoparticle suspensions are exposed to the magnetic field, the separation of the aqueous phase is almost complete (> 90%) for solutions with Zn, Na and Ca (Fig. 9).
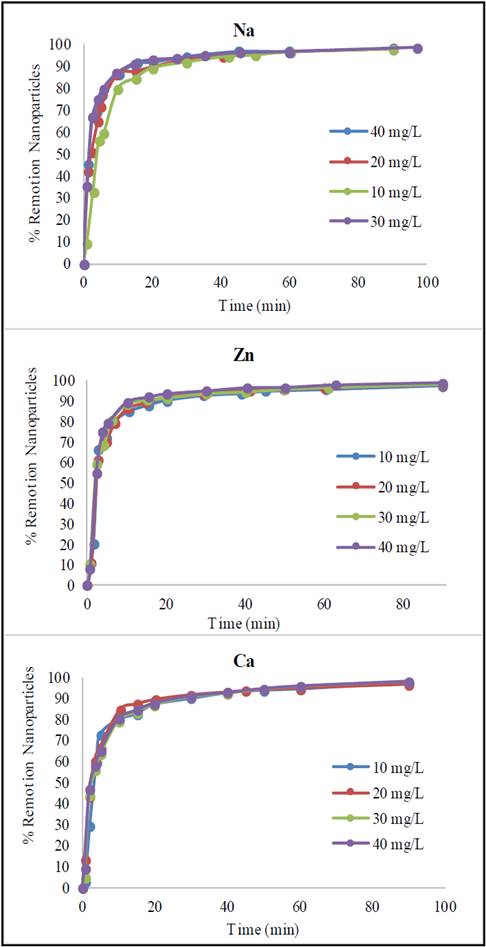
Source: The authors
Figure 9 Sedimentation dynamics of Glutathione@MNPs in aqueous suspension in a magnetic gradient in the presence of ions at different concentrations.
The increase of sedimentation velocity in the presence of salts with charge 1+ and 2+ in the presence of magnetic field, especially in the initial period, is evidently related to the strengthening of aggregate formation processes. The dependence of the sedimentation rate on the concentration of salts in the suspensions gives evidence of the change in the electrostatic surface charge of the particles, which is confirmed by the change in the zeta potential of the particles in the presence of the salt additives. The fact that calcium ions promote a greater increase in sedimentation rate is due to its higher ionic charge (2+ compared to Zn and Na which is 1+), as well as the possibility of creating additional bonds (since it is of the bridge group type) between the particles of Glutathione@MNPs, accelerating the aggregation process [34].
With this result, there is the possibility that triply charged cations further strengthen the aggregation of particles in the suspension, which would be an open study area for future work.
These results are quite encouraging given that, for an implementation of the magnetic material in water treatment operations, some of these salts can be added to obtain a more efficient separation and to remove the nanoparticles from the effluent and thus not have a release to the environment that can Affect ecosystems or human health.
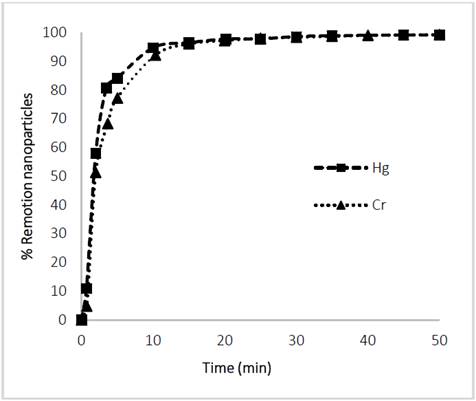
The separation rates of Glutathione@MNPs after performing the heavy metal (Hg and Cr) adsorption processes for 120 min was evaluated. It can be seen from Fig. 10 that the separation of the heavy Nanomaterial-Metal assembly is effective since more than 90% is withdrawn from the aqueous phase. This is because the active adsorption sites such as thiols, amines and carboxyl groups react with the metal ions of Hg and Cr reducing the effective surface charge and consequently the steric forces that provide the stability leading to an increase in the ease of separation by means of the magnetic field [40- 42].
The above results provide the possibility of creating water treatment systems for heavy metal remediation with MNPs, as it gives control of non-release of nanomaterial or heavy metal into the body of water.
4. Conclusions
Glutathione@MNPs show a stability in aqueous medium with no presence of ion metals over 8 weeks. This stability is due to the steric repulsions of the carboxyl, amine and thiol groups on the surface of the material.
This stability was reduced by the presence of valves in calcium, zinc and sodium, where an increase of more than 500% in the average hydrodynamic radius of the nanoparticles was found.
If the critical concentrations of coagulation come from the application of the DLVO theory, where calcium sales consume the lowest concentration to promote the agglomeration of the particles and that the charge of these emulsions is mayor in comparison of air of zinc and sodium.
The application of magnetic field for the separation of the particles of the aqueous medium is effective, since with the fields of 0.2T a separation greater than 90% of the material was reached. Also, for nanoparticles bound to heavy metals such as the Hg and Cr son, they do not inhibit the separation of material from the aquatic medium, which gives indications for the use of this material for environmental remediation.
The above results provide the possibility of creating water treatment systems for heavy metal remediation with MNPs, as it gives control of non-release of nanomaterial or heavy metal into the body of water.