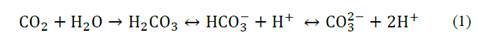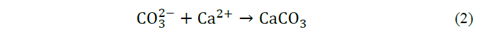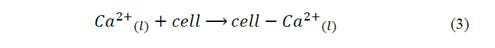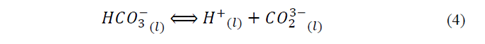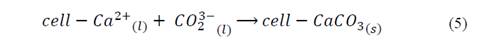1. Introduction
The biomineralization of calcium carbonate is a process associated with eukaryotic and prokaryotic physiologies. In the bacterial world, many microorganisms are capable of precipitating calcium carbonate crystals in the form of calcite or vaterite [1]. The bacteria of the genus Bacillus spp. (mainly Bacillus subtilis, Bacillus pasteurii, Bacillus amyloliquefaciens, Bacillus cereus) are ones of the most widely known. This microorganism require CO2 dissolved in the medium (in the form of HCO3 2- and CO3 2-) and a defined source of calcium (generally as a salt) to carry out the biocrystallization of calcium carbonate [2]. It is believed that the microorganisms produce an induced biomineralization (activated), where the bacteria regulate the calcium entry and exit through of a protein-non protein channel. The Ca2+ ions expelled by the bacterium in presence of CO3 2- facilitate the formation of calcium carbonate [3,4].
The ability to precipitate calcium carbonate crystals is continuously studied, because of its multiple applications in the industries of construction, bioremediation, medicine, cosmetics and others [5,6]. It is noteworthy that the formation of calcium carbonate is a complex process that can be manipulated in several ways. The control of the morphology and the size of the crystals are very important, considering its final application. The kind of the obtained mineral depends on several conditions of the medium, including temperature, saturation, pH as well as the presence of additives and impurities [7].
In general, the culture media employed in the induced biomineralization processes of calcium carbonate require a calcium salt (usually calcium acetate) and a nutritious broth, composed of various components (glucose, yeast extract, tryptone, etc.) [8-10]. In preliminary trials for this work, it was found that a strain of Bacillus cereus was capable of producing calcium carbonate from a medium that only contained 1.0% of tryptone and 0.5% of calcium acetate (data not shown).
This work evaluated the effects of reducing the tryptone concentration on the carbonate bioproduction to determine a minimum concentration of the compound that can allow an adequate carbonate precipitate index, considering a scalable process at a commercial level with a lower reactive consumption. All the obtained precipitates were mineralogically analyzed to observe if there were morphologic and or polymorphic variations.
2. Experimental procedure
2.1. Microorganism
A pure culture of Bacillus cereus was selected for the experiments. It was isolated from soil material from the gardens of the Universidad Pontificia Bolivariana (Medellin, Colombia). The strain was adapted prior to the calcium carbonate biomineralization process. The adaptation consisted in several subcultures in a modified M3 medium (0.5% calcium acetate and 1.0% tryptone) up to observe no variations on pH and the quantity of precipitate generated between the replicas.
The inocula were prepared in a 250 mL erlenmeyer, using different concentrations of tryptone for each bacterial assay (from 0.2% to 1.0%). The working volume was 100 mL and the culture composition had a ratio of 1 mL of inoculum per 9 mL of M3 modified medium (evaluating the respective tryptone concentration) [9]. The strains were incubated for 6 days in an orbital agitator at a temperature of 30°C ± 1°C and an agitation velocity of 180 rpm ± 2 rpm.
2.2. Biomineralization of calcium carbonate
Each assay was prepared in a 250 mL erlenmeyer (working volume of 100 mL), using a ratio of 1 mL bacteria inoculum per 9 mL or modified M3 modified medium. The variable evaluated was the tryptone concentration (%w): 0.2, 0.4, 0.6, 0.8 and 1.0.
All assays were incubated for 6 days under temperature and agitation conditions like the used in the preparation of the inocula. The experiments also had their respective replica and abiotic control (without presence of microorganisms). Additionally, the pH was periodically monitored for each sample, using an HACH HQ 30d pH meter.
At the end of each assay, 80 mL were extracted from each erlenmeyer and centrifuged at 3500 rpm for 10 minutes. After this time, the supernatant was discarded and the precipitate was washed 4 times with distilled water and other 4 times with ethylic alcohol (90 %v), agitating in a vortex for 1 minute and then centrifuging at 3500 rpm for 10 minutes. Afterwards, the samples were dried at room temperature. The final product was used to determine the amount of formed precipitate per liter of medium, dividing the obtained weight over the sample volume.
2.3. Mineralogical analyses
The mineralogical composition of the samples was established with X-ray diffraction (XRD), Fourier transform infrared analysis (FTIR) and scanning electronic microscopy (SEM). For all the analyses, the original sample was used, without particle size transformations.
The XRD analyses were made in a Rigaku Miniflex II diffractometer, using the step by step method, with 5° start angle, 70° stop angle, 0.05° step angle, and counting time of 50 s. The minerals present in the precipitate were quantified using a Rietveld refinement in the X’Pert HighScore Plus© software with the PDF-4-2012 database and the American Mineralogist Crystal Structure Database [11].
The FTIR analyses were carried out in a Thermo Scientific Nicolet spectrometer, using the ATR method with the pure sample, using an interval of 400-4000 cm-1, 32 scans and a spectral resolution of 4 cm-1.
For the SEM analyses, the sample was coated with a fine silver layer. The “sputtering” (cathodic pulverization) coating technique was performed with a DENTON VACCUM DESK IV. The analysis was carried in a JEOL JSM-6490LV sweep electron microscope. Additionally, an Oxford Instruments INCA PentaFETx3 X-ray-EDX analyzer was employed to carry out the microchemical sample analyses.
3. Results and discussion
3.1. Biomineralization of calcium carbonate
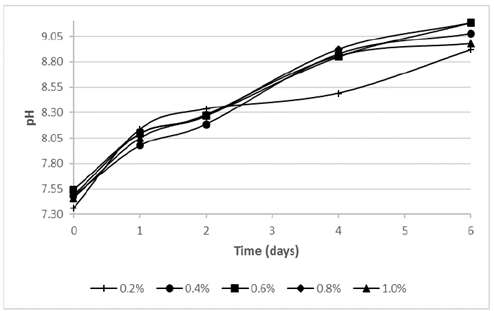
Fig. 1 illustrates the pH behavior, where all the assays had a continuous increase of the value. Probably, the bacterial growth could have affected this parameter, because Bacillus cereus is heterotrophic and it is able to carry out the aerobic ammonification process of amino acids in presence of dissolved oxygen, organic matter and calcium [6].
Source: The authors.
Figure 1 pH vs. time during the calcium bioprecipitation process at different tryptone concentrations.
According to Periasiami et al. (2016), the microorganisms might be able to produce carbonate compounds (CO3 2−) during its cellular growth and metabolism activation. This compounds might have reacted with Ca2+ ions (from calcium acetate), affecting the acid-base environment in the culture medium [12].
Although the assays using a tryptone concentration ≥ 0.4% tended to stabilize at day 6 of the process, the assay using 0.2% of tryptone decreased its raising from the second day. The final pH for all assays were around 9 and the highest values were achieved by the assays using tryptone concentrations of 0.6% (9.19) and 0.8% (9.18), followed by the 0.4% (9.08), 1.0% (8.98) and 0.2% (8.92) tests.
The differences among pH tendency can be explained by the availability of energy source. A high tryptone concentration might imply a higher access to proteins necessaries to bacterial multiplication, giving as result a higher carbonate compounds and therefore alkalization of the medium [13,14].
Fig. 2 presents the quantity of precipitate obtained in each assay. The assays with tryptone concentration higher than 0.4% obtained values above 3.2 grams of precipitate per liter of solution. The highest precipitate quantity was obtained in the assays with 0.8% (3.56 g/L) and 1.0% (3.58 g/L). In contrast, the assay using 0.2% tryptone obtained the lowest precipitation, 44.5% lower than the experiment with the subsequent precipitate concentration (3.23 g/L).
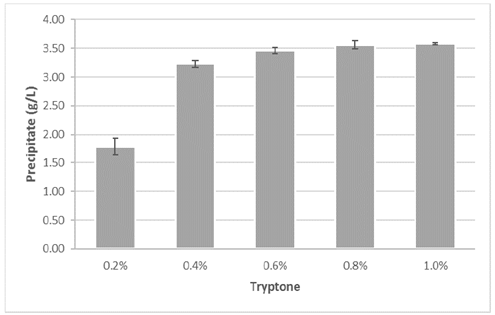
Source: The authors.
Figure 2 Obtained precipitate during the calcium bioprecipitation process at different tryptone concentrations.
The precipitation of calcium carbonate observed in the assays using the highest concentrations tryptone (Fig. 2) might be related with the propagation and metabolism of the microorganism which promote the mineral synthesis, either segregating metabolites which accelerating CO2 hydration, regulating CO2 y HCO3 - concentrations and modifying pH of the medium, as shown in eq. (1) and eq. (2) [15,16]. For the last reason, calcium carbonate biomineralization is product of the quantity of CO3 2− and Ca2+ ions in the medium.
3.2. Mineralogical analyses
Fig. 3 shows the diffractograms of the precipitates for all the assays using different tryptone concentrations. The presence of calcium carbonate was identified in two crystalline phases: vaterite and calcite, where vaterite was the predominant polymorph. Notwithstanding, the assays using 0.2% and 0.4% of tryptone presented a greater intensity in the calcite peaks in comparison to the other experiments. On the other hand, none of the assays presented formations of other minerals.
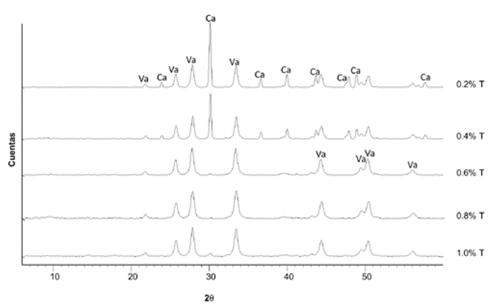
Source: The authors.
Figure 3 Diffractograms of the precipitates for each trial at different tryptone (T) concentrations. Va: vaterite, Ca: calcite
From Rietveld refinement of the XRD patterns (Fig. 1), Table 1 presents the semi-quantitative ratios of the crystalline phases of both vaterite and calcite for all the precipitates. The weighted residual profile of the refinements (Rwp) was below 10% which did not vary more than 20% with respect to the residual expected (Rexp). These parameters determined that the refinement models were good to accept the values obtained [17-19]. The assays with the three highest tryptone concentrations had a greater vaterite formation (above 97%), while the percentage of calcite was increased considerably when concentrations below 0.4% tryptone were used.
Table 1 Percentage of calcite and vaterite in crystalline phase of the obtained precipitates in the tests at different tryptone concentrations.
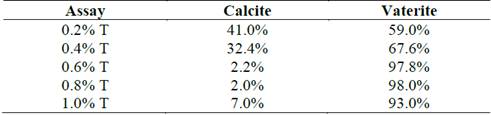
Source: The authors.
Wei et al. (2015), explain that the polymorphism of carbonate biomineralization to calcite, vaterite or aragonite depends of the environmental conditions and the culture medium. Additionally, different types of bacteria can precipitate calcium carbonate with diverse crystalline structure (spherical or polyhedral) or even amorphous forms [20].
Bacillus cereus is an ureolytic bacterium which promotes the production of calcium carbonate when the culture medium is enriched with a protein source, as tryptone. For this purpose, the microorganism activates the biochemical metabolism of the enzyme urease [1]. Although vaterite is the less stable among the three polymorphs of anhydrous calcium carbonate at environmental conditions and it tends to transform into calcite, several research have found stability of bio-vaterite by biomolecules from the cellular wall of the bacteria, where compounds with phosphorus ions, carboxylic groups or polysaccharides play an important key role to coordinate calcium and carbonate orientation [1].
Based on the results of this work, the reduction of the main nutrient source below 0.4% probably reduce the synthesis of the biomolecules that maintain the stability of vaterite, thus it might have allowed a transformation of a part of calcium carbonate into calcite.
Fig. 4 shows the FTIR spectra for all the precipitates and Table 2 presents the bands identification with their respective assignations. The presence of calcite and vaterite was validated for all assays. The band at 713 cm-1, corresponding to calcite, indicated the greatest presence of this mineral in the assays with 0.2% and 0.4% of tryptone. The bands at 1280 cm-1 and 1707-1725 cm-1 indicated that the precipitates had traces of organic matter after the washing phase, probably originating from one of the compounds of the cultivation medium. The band at 1648-1653 cm-1 signaled the presence of natural humidity of the sample and the band at 3282-3300 cm-1 indicates interactions of O-H, possibly corresponding to the to the residual alcohol from the washing phase of the precipitates [21,22].
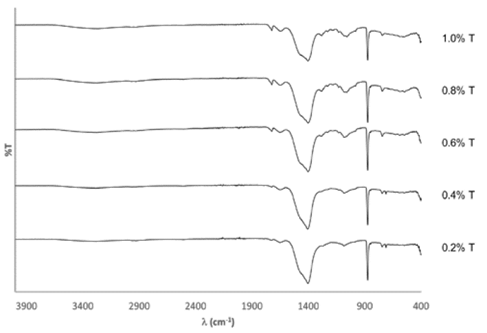
Source: The authors.
Figure 4 FTIR spectra of the precipitates for each test at different tryptone (T) concentrations.
Table 2 Bands of the FTIR spectra for the obtained precipitates at different tryptone concentrations.
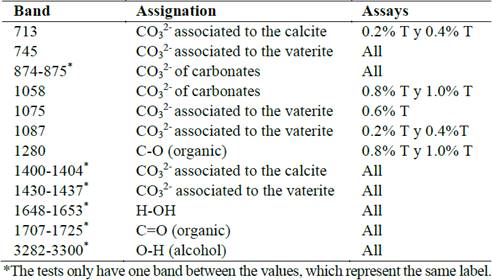
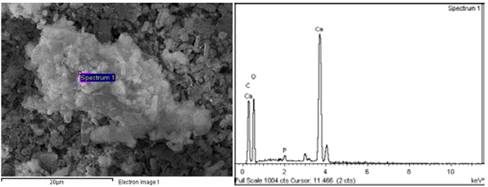
Fig. 5 illustrates SEM images. All the precipitates presented a cylindrical, hollow structure formation pattern with an external diameter of approximately 1 µm and variable lengths between 5 µm and 15 µm, possibly originated from the biomineralization of calcium carbonate nanoparticles over the microorganisms. Probably, the bacteria had a surface with charged nucleation sites where the mineral could initialize its generation [23]. The samples of the assays with the highest tryptone concentrations (Fig. 5d) also presented other typical structures, including spherical particles which correspond to the formation of vaterite [29]. Other formations founded were ramified carbonate less than 1 micron (Fig. 5h), product of particles that did not probably tend to form the shells of microorganisms. The accumulation of some particles (Fig. 5i) could be attributed to the washing phase of the precipitates.
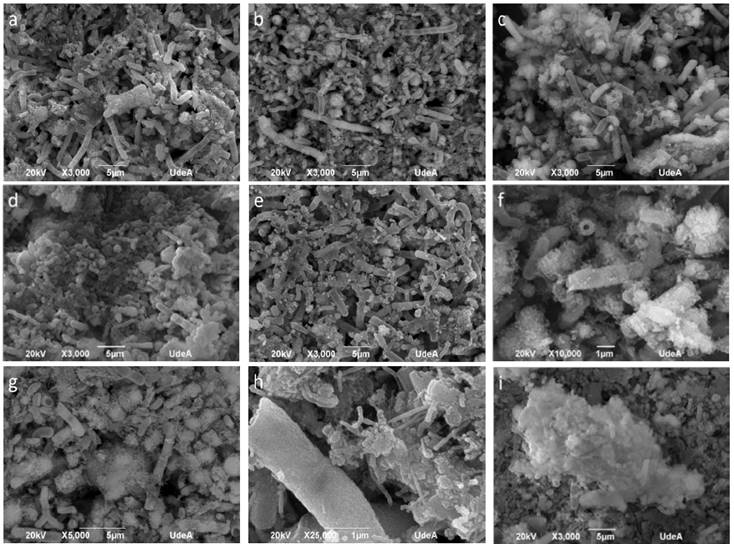
Figure 5 SEM images of the precipitates of the tests at varying tryptone concentrations. Formation of bacillary structures from the biomineralized microorganisms of the tests 0.2% T (a), 0.4% T (b), 0.6% T (c) and 1.0% T (e). Accumulation of vaterite spheres in the 0.8% T (d) test. Magnification of the precipitates of test 0.4% T which indicates that the cylindrical structures are hollow (f). Coating of the shells with stem shaped mineral growths (g and h) and accumulation of agglomerate particles (i).
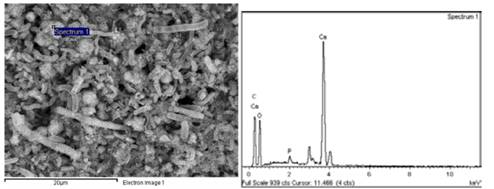
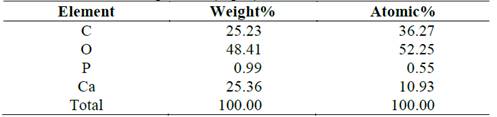
From the SEM and EDS analyses, microchemical analyses were carried out to some of the structures found in the precipitates (Fig. 5). Fig. 6 and Table 3 illustrates the particles that covered the biomineralized shells of the microorganisms (assay 0.4% T), where a low calcium concentration and a high carbon concentration was identified. Comparing with the ideal atomic relations of calcium carbonate (1 Ca:1 C:3 O), it indicates that the particles or their surroundings presented components of organic matter possibly from precipitated organic compounds, product of the reaction mechanisms of the microorganisms or traces of the bacteria that were within the structures.
Source: The authors.
Figure 6 EDS of the particles that coated the shells of the biomineralized microorganisms (test 0.4% T)
Table 3 Microchemical analysis of particles that coated the shells of the biomineralized microorganisms (Fig. 6).
Source: The authors.
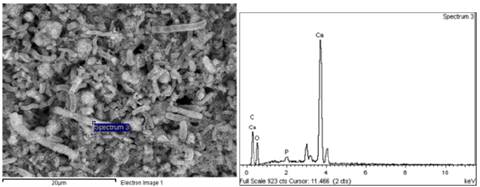
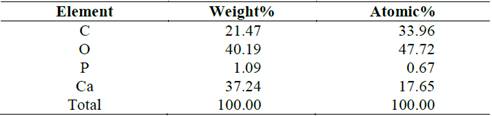
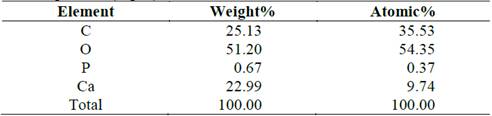
Fig. 7 and Table 4 shows the microchemical analysis of the surface of a shell (assay 0.4% T). The highest calcium peak indicates a higher purity in the structure. Notwithstanding, the high carbon content indicates the presence of residual organic matter in the surroundings.
Fig. 8 and Table 5 shows the microchemical analysis of an accumulation of particles (assay 0.4% T). As was observed in Fig. 6 and Fig. 7, the high amount of carbon indicates the presence of residual organic matter in the surroundings.
The mineral formation over the bacterial surface could have presented in two stages:
Source: The authors.
Figure 8 EDS of shell accumulations of the biomineralized microorganisms (test 0.4% T).
Table 5 Microchemical analysis of shell accumulations of the biomineralized microorganisms (Fig. 8).
Source: The authors.
The possible reaction in the nucleation sites mediated by Ca2+ ions by the mechanism showed in the eq. (3) to eq. (5) [33]. In first place, the metallic ions from the medium might have stoichiometrically interacted with the charges groups of the bacterial surface structures, allowing the mineral deposition and generating bacillary walls, as observed in Fig. 5 to Fig. 7 [34].
After precipitation, the second stage implies the increasing of the calcium carbonate concentration and saturation of the cell surface, generating an agglomeration and precipitation outside the cell, as shown Fig. 8. This precipitation is not controlled by the microorganism, but by the physic-chemical environment of the surface around each bacterium.
On the other hand, the traces of phosphorous identified in the microchemical analyses (Table 3 to Table 5) can have their origin in some of the components present in the Tryptone of the cultivation medium.
4. Conclusions
The microorganisms used in the project had a greater tendency to produce calcium carbonate nanoparticles as vaterite. The ratio of vaterite-calcite and its degree of purity can be affected by the tryptone concentration. However, a tryptone concentration below 0.4% would limit the metabolism of the bacteria and therefore the CO2 formation, which could cause a deficit in the production of the precipitate. Notwithstanding, a 0.4% concentration produced a good precipitate rate, which is comparable to the results obtained at higher concentrations.
The processes carried out allowed the formation of shell shaped structures, which are composed of calcium carbonate nanoparticles (vaterite-calcite) with a bacillary morphology and hollow core, which could be considered as “microbricks”. Because the shells do not collapse after the washing phases of the precipitate, these could have potential applications in the manufacture of osseous structures for the microencapsulation of medications due to their hollow and almost uniform distribution.