1. Introduction
In developing countries, the majority of sources for drinking water supply systems are affected by natural phenomena and anthropogenic effects that deteriorate their quality [1]. Limitations on the efficiencies of the production processes result in drinking water that complies with established quality standards [2-4]. Various treatment technologies, which use several combinations of processes, are available for the purification of surface water, including coagulation/flocculation, sedimentation, filtration, disinfection and pH stabilization [2,5,6].
Filtration is a process that is always used in water treatment technology. It consists of the separation or removal of particles in a liquid by flowing through a porous bed [2,5]. Conventional treatment technology with sand or sand and anthracite rapid filters has allowed compliance with Colombian Resolution 2115 of 2007, which established a maximum permissible turbidity in drinking water of 2 NTU [7]. However, several authors recommend that to ensure the minimum risk of drinking water, the turbidity of the filtered water should be in the range of 0.1 to 0.5 NTU to eliminate or deactivate pathogenic microorganisms [3,8,9].
Several typical filtration systems do not have the ability to effectively remove turbidity and dissolved organic matter, and it is necessary to evaluate other treatments to improve the quality of drinking water [3]. Double filtration is a treatment technology that consists of a first stage of filtration in which the water flows through a granular medium with a high capacity of solids removal. This process reduces the turbidity and is followed by a second stage of filtration for polishing [10]. In the last stage, mineral or vegetal GAC has been used as a filtration medium for water purification due to its characteristics, which allow it to remove turbidity and dissolved organic matter and increase the treatment efficiency [11-14].
Turbidity is a key parameter for evaluating the efficiency of filtration because it is simple and rapid to quantify, and it is indirectly related to the particles in the water, which in turn are associated with bacteria, protozoa and viruses [15,16]. Organic matter is associated with aesthetic impacts on water quality, with the formation of disinfection byproducts that may become carcinogenic and/or with synthetic organic compounds (e.g., pesticides, personal care products, pharmaceuticals) that are difficult to remove with conventional drinking water treatments [6,17].
GAC is one of the most widely used adsorbents in water treatment [18]. According to Wiecheteck et al. [19] and Silva et al. [20] double filtration has several favorable characteristics, such as greater solids retention and a more effective response in the removal of organic matter. Authors such as Bundy et al. [21] have used conventional sand and anthracite filtration and secondary filtration with GAC and achieved a turbidity reduction to less than 1 NTU and a removal efficiency of pharmaceutical compounds on the order of 95%. Silva et al. [20] also used GAC to eliminate cyanobacteria, color, organic matter and halogenated organic by-products. Pham et al. [22] used coconut husk GAC and obtained removal efficiencies of turbidity (97%) and organic matter (COD:68%) and a pesticide adsorption capacity greater than 50%. Thiel et al. [17] concluded that sand:GAC filters are effective at removing precursor organic matter from disinfection products and generate effluents with turbidities less than 0.3 NTU.
Based on these investigations, this study evaluates at laboratory scale, the secondary filtration with different proportions of mineral and vegetal activated carbon using filtered water from a conventional water treatment plant in the city of Cali. The second filtration was evaluated to determine its effect on the removal of turbidity and dissolved organic matter, which were measured as UV254.
2. Matherials and methods
2.1. Experimental unit
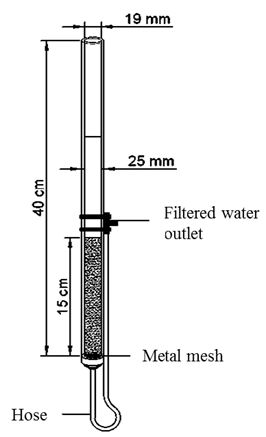
Twenty-four laboratory glass filters with a nominal diameter of 25 mm, an internal diameter of 19 mm and a length of 40 cm were used. The lower part of the filter was supported by a metal mesh to prevent clogging of the filter outlet and loss of the filter medium, which was 15 cm high [5,23,24]. To provide a hydraulic load to ensure that water was present throughout the filter medium, the effluent was collected through a silicone hose attached to the bottom of the filter. The other end of the hose was located above the upper part of the filter medium [5]. The filtration columns were placed in a support structure, which allowed proper operation of the filters (Fig. 1).
The experimental unit was fed by a multi-partition distribution system. Gravity flow was distributed to each laboratory filter through silicone hoses, which had flow control valves at their ends to allow the flow to be distributed through constant dripping to the filters. This distribution system was supplied from a temporary storage tank in which the water level was kept constant to avoid variations in the inlet flows to the filters.
2.2. Water used in the study
The laboratory-scale study used filtered water from the Puerto Mallarino WTP, which had undergone coagulation, flocculation, clarification and downstream sand and anthracite filtration [25,26]. Table 1 shows the characteristics of the filtered water.
2.3. Filter media
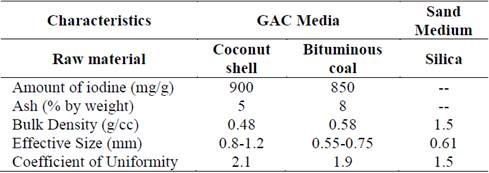
The characteristics of the GAC and sand filter media used in the test are given in Table 2.
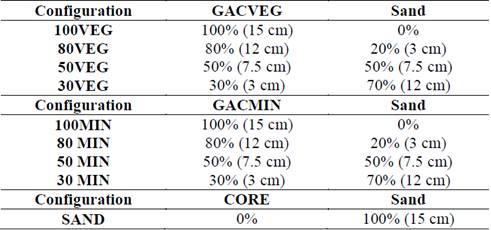
The configurations correspond to the height proportions of the filter media that consist of GACVEG and sand, GACMIN and sand and only sand. These configurations are presented in Table 3 and were analyzed in triplicate.
2.4. Operation of the experimental units and process monitoring
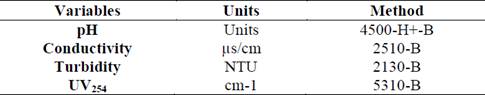
Filtered water from the WTP, was distributed to each laboratory filters at a constant filtration rate of 61 m3/m2d for a flow rate of 12 ml/min and a filtration time of six hours [5, 24]. The follow-up variables are shown in Table 4 and were measured every 5 minutes during the first half hour of filtration. Subsequently, they were measured at 10 minute intervals for the remainder of the first three hours and every 15 minutes in the last three hours of the test. The physical and chemical variables were measured according to the guidelines established in the standard method [27].
3. Results and discussion
3.1. Influence on turbidity removal
Turbidity is an indirect parameter that indicates the potential of microbiological risk in drinking water. Low values of turbidity in filtered water (<0.30 NTU) indicate greater efficiencies in the removal of protozoa (Giardia and Cryptosporidium) during filtration and favor the elimination of bacteria and viruses during disinfection [15]. Fig. 2 shows the behavior of the turbidity during the test for the two types of carbon (vegetable/mineral) and sand with their respective filter media configurations.

Source: Los Autores
Figure 2 Behavior of the turbidity over time for the two types of activated carbon and sand with their respective configurations.
Fig. 2 shows that all of the media configurations resulted in similar turbidity behavior as a function of time. The turbidity values are below the initial turbidity value obtained in the first filtration stage. As indicated by Wiecheteck et al. [19] and Silva et al. [20], double filtration provides greater turbidity removal than conventional filtration.
Ninety-seven percent of the data for all configurations resulted in turbidity values less than 0.3 NTU, which is the threshold value of the WHO [2] and the EPA [15] before disinfection to eliminate chlorine-resistant pathogens and ensure the effective elimination of Giardia, Cryptosporidium, and other material. All of the turbidity values measured for each configuration in this study complied with the national regulations for drinking water (<2 NTU) [7].
Graese et al. [28] founded turbidity <0.2 NTU in the effluent with GAC filtration, which is consistent with this study. The median values for the filters with the GACVEG configurations were between 0.199 and 0.238 NTU, those for the GACMIN filters were between 0.206 and 0.236 NTU, and those with sand had the lowest value of 0.181 NTU.
The median values obtained for each configuration show that the configuration with the sand filter resulted in the best reduction of turbidity; however, all configurations with GAC provided effective turbidity removal.
Although the GAC configurations showed lower turbidity removal than the sand filter, these results demonstrate that the implementation of activated carbon ensured the quality of the final effluent.
3.2. Influence on the removal of organic matter measured as UV 254
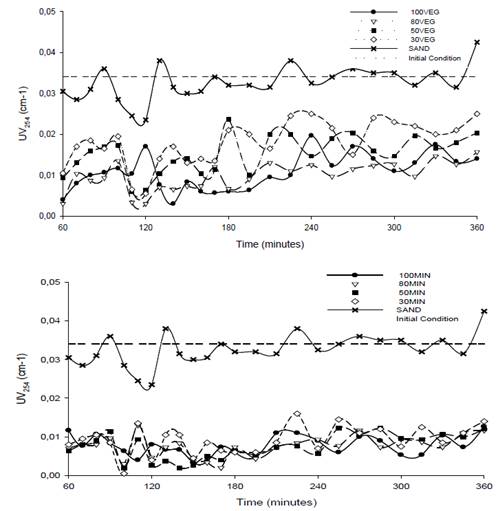
Organic matter is an important constituent of water that affects the performance of treatments in drinking water processes and the quality of drinking water. As a result, it requires the extensive use of coagulants, oxidants and disinfectants in addition to being a precursor to the formation of disinfection byproducts [29]. Several studies have used the UV254 absorbance test as a parameter to evaluate the efficiency of the GAC filtration process for the removal of dissolved organic matter, which is capable of absorbing UV light [6, 20, 29]. Figure 3 shows the behavior of UV254 during the tests for the two types of carbon (vegetable/mineral) and sand with their respective filter medium configurations.
Fig. 3 shows that the configurations with GACMIN and GACVEG were below the UV254 absorbance value reported in the first filtration stage, whereas the sand filter remained very similar to the initial UV254 value. Similar results were obtained by other studies, which concluded that sand does not effectively remove compounds associated with organic matter [30,31].
Source: Los Autores
Figure 3 Behavior of UV254 over time for the two types of activated carbon and their respective configurations.
Median values of UV254 between 0.010 and 0.019 cm-1 were obtained with the GACVEG configurations, median values between 0.007 and 0.008 cm-1 were obtained with the GACMIN configurations, and a median of 0.032 cm-1 was obtained with the sand. The results indicate that the sand did not significantly affect the reduction of organic matter because its values oscillated near the initial absorbance of 0.034 cm-1, and the configurations with GACMIN had a greater effect. The GAC filters clearly yielded a reduction of organic compounds as measured by UV254. Therefore, the use of granular activated carbon as a filter media is recommended not only for reducing odor and flavor but also in the adsorption of organic compounds [9].
Silva et al. [20] demonstrated the removal of UV254 using GAC in double filtration, and Pham et al. [22] achieved removal efficiencies of organic matter greater than 68%. These results were similar to those found in this study; the removal percentages for the GACVEG configurations ranged from 46% to 72%. The GACMIN configurations resulted in the highest removal percentages, between 75% and 78%, whereas the sand configuration only removed 6% of the organic matter.
The results indicate that filters with GAC in double filtration technology can remove organic substances, which reduces the possible risk associated with the formation of disinfection byproducts [17,32].
Additionally, Kim and Kang [33] found that replacing sand filters with dual-medium GAC-sand filters represents an ideal choice for the removal of organic matter compared to turbidity removal. This was confirmed by the results of this study, which showed small differences in the turbidity decrease between the GAC and sand configurations, whereas the configurations of the GAC filters eliminated organic matter more effectively than the sand filter alone.
4. Conclusions
Although the sand filter achieved a higher turbidity removal efficiency, all configurations with GAC provided effective removal that complied with the limit of 0.3 NTU recommended by the WHO and EPA to mitigate microbiological risk and with the limit of <2 NTU provided by Colombian Resolution 2115 of 2007.
For organic matter, which was measured as UV254, all configurations containing GAC were more efficient than the sand, which removed only 6% of the organic matter. A greater proportion of GACVEG resulted in a greater percentage of reduction of UV254 in the effluent (100VEG: 70%, 80VEG: 70%, 50 VEG: 53%, 30VEG: 46%), and GACMIN obtained similar and higher efficiencies than GACVEG (100MIN: 78%, 80MIN: 77%, 50MIN: 77%, 30MIN: 75%).
Although Resolution 2115 of 2007 does not provide a maximum allowable amount of organic matter in drinking water in terms of UV254, the removal efficiencies for organic matter of the configurations with GAC were greater, which confirmed that GAC is suitable as a filter medium not only for the reduction of odor and flavor but also in the adsorption of organic compounds.
Double filtration has several favorable characteristics, including a greater retention of solids and a more effective removal of organic matter, which make it an efficient technology to ensure the quality of the final effluent, minimize risk and reduce the limitations in the treatment of water for human consumption.