1. Introduction
Elements such as patient´s safety, security in the supply chain, traceability and efficiency in the health care sector, are nowadays at the top of the list of the most relevant issues in the regulations and industries of world governments [1-3]. Various situations have shown the existence of product safety crises in different countries around the world, for example, among the many cases related to illicit drugs, it is estimated in a range of 3.8% to 8.9% of deaths due to malaria-related diseases in Sub-Saharan Africa due to low-quality or warped drugs [4].
The World Health Organization (WHO) has received reports of illicit drugs from many regions of the world, this shows how global and serious the problem is [5], Fig. 1 shows the main countries from where reports were issued between 2013 and 2017.

Source: WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products [5].
Figure 1 Countries from where reports of low-quality or counterfeit medicines were sent to the WHO between 2013 and 2017 (dark color).
The regions where the highest incidence of illicit drugs is reported are Central America, South America, Sub-Saharan Africa and India. In these the figures of warped drugs are superior to 20% and go too far up to 50% of the traded medications. The regions of lowest incidence are North America, Europe, Japan and Australia in a range from 1% to 10% [6].
Many reasons can cause the excessive spread of illicit drugs, among them selling through Internet, high cost of patent medications, lack of health policies and the complexity of supply chains [5-7].
A vital strategy for companies must be keeping sustainably competitiveness and offering the best service to the customer; for this, the best methods and tools must be continuously adopted, such as the implementation of traceability systems that allow them to meet the goal [8-10]. These systems involve confronting illegal activities for avoiding illegitimate drugs and the elimination of manual information management procedures, which create doubly manipulation and costly reprocessing, limiting an optimal negotiation among supply chain partners.
In the health sector, as a part of its drugs traceability, it is vitally important to always obtain veracious and reliable information about each product, medicine or service; for this, it is necessary to achieve the unique identification that allows all laboratories, drugstores and pharmacies to use the same communication language, regardless of any distribution channel used or final destination place of goods. The use of GS1 standards for implementing successful traceability systems is proposed as a good solution, since they provide global, interoperable and open results for optimizing the logistics system [11].
The present investigation is focused on the study of the traceability system of a Cuban 3PL drugs Logistic Service Provider (LSP) which is responsible for managing the distribution of medicines throughout all the country, ensuring their availability in health centers and communal pharmacies.
This 3PL has a national coverage and its main difference with other globally existing 3PL in the sector is that it covers 100% of the company´s drugs distribution to all pharmacies, hospitals and other healthcare centers in the country, while in other environments some known 3PLs attend only a part of the market. Due to this characteristic, any proposed solution has an impact on the entire drug system.
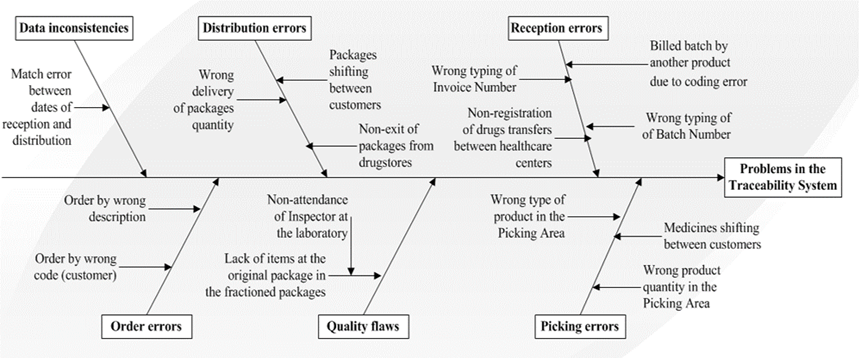
Previous studies carried out in the company using the Inventory Reference Model tool called by its Spanish acronym MRInv [12], have shown the existence of problems in products coding that affect the management of inventories and traceability systems: the results of quality claims, the pretends and real drugs called back from the market, gave evidence of a series of deficiencies that represent failures in the traceability system. All these errors and/or deficiencies can be evidenced in the Ishikawa Diagram shown in Fig. 2.
All previously evidences the need to solve the existing deficiencies in the company's traceability system. Thinking of traceability as a system, the aim of this article is to propose a systemic solution for innovating the Traceability System of a drugs 3PL LSP, based on the use of GS1 international standards.
It’s necessary for drug Logistics Service Providers to design their traceability plan, supporting the identification of items under the premise of unique identification using proposed GS1 standards. This result can be widespread to several logistics operators.
2. Materials and methods
The research was carried out in a 3PL Logistic Service Provider by performing a diagnosis of the trading and distribution current situation in what concerns drugs identification and traceability. For this, methods such as interviews, direct observations, analysis of technical documents, data processing and resolutions and norms in effect internationally and in Cuba were used. Traceability exercises consisting of following the entire product route throughout its logistics cycle and monitoring activities by tracking the information in the computer systems used in the chain of supply were also carried out, identifying the interoperability of the systems.
In addition, the Products Coding and Classification Systems Module from the Inventory Reference Model was evaluated using the MRInv tool [13], obtaining the main deficiencies of the company in terms of medicines identification.
The main tools used during the investigation were: The Ishikawa Diagram and the V-63 Visualization Pattern. The first one is used to show the current problems of the traceability system in the company and the second one to analyze the information flows among the existing business applications.
The V-63 Visualization Pattern is a part of the Entrepreneurial Architecture Management Patterns that help to manage the information of business applications in an agile and structured way [14].
3. Results
3.1. Regulatory domain
The analysis of the traceability system of the organization begins with the study of the existing environment that regulates Traceability in Cuba in what concerns health sector.
The main legal regulations analyzed [15-20] show that the Center for the State Control of Drugs, Equipment and Medical Devices, CECMED in its acronym in Spanish, establishes the necessary documentation and procedures to guarantee traceability, but it does not specify neither how to be done nor the essential rules to carry it out. To this effect, the Resolution 117/2008 [17], previously issued by an inexistent present organ and currently obsolete, is the most advanced normative as it recognizes the existence in an incipient way of the bar codes in the chain and establishes procedures and registers aimed to guarantee traceability; however, it is still applied by companies because it provides them with methods to comply what´s been established by CECMED. There is a Joint Resolution 1/2001 [18] signed by Ministry of Domestic Trade and Ministry of Foreign Trade that establishes the use of coding standards, for which the GS1 Office in Cuba was constituted, however, it doesn’t express a procedure for the implementation of these standards in Cuban companies. On the other hand, Resolution 11/2007 of the Ministry of Finance and Pricing [19] indicates the obligatory documents and information fields that help to guarantee traceability, but it does not mention the use of an internationally standard code.
The regulations issued by the CECMED regarding the post-merchandising surveillance of the products, in spite of including topics such as monitoring to combat illicit drugs and the calls back from the market, do not refer to the existence of traceability systems to support these regulations [15].
In general terms, a delayed regulatory framework regarding to foreign regulations that exist for the health sector in terms of traceability is observed, since work with the GS1 standard codes is not mandatory to have an open, interoperable and global system and there are no master documents that guide the implementation and evaluation of traceability systems in Cuba for the sector.
3.2. Elements that make up the current traceability system
The execution of traceability exercises and monitoring activities through informatics systems allowed knowing the elements that make up the current traceability system and tracing the drugs in the supply chain. The provincial drugstores in which the study was done at this stage, not extended to hospitals, clinics or local pharmacies, allowed to understand the functioning of the current traceability system, being the basis for the design of improvements.
The elements that make up this system are presented below, specifying in each case the type of organizational unit in which they are found:
Actors: involved in the traceability process are the Informatics Department, Head of Operations, Claims Department, Technical or Quality Department, Economy Department, Warehouse Head and Dependents where the product is receipted, Driver of the distribution vehicles, inspection and storage personnel that receives the goods at the customer's point.
Documentation: Entry Registers, Entrance Inspection, Entry of products with special conditions, Temperature and Humidity Control, Batch and Expiration Date Control, Control of Expedition Guides, Control of the Cold Chain of products from 2-8°C, Supplier Expedition Guide, Vendor Invoice, Ownership Transfer among areas, Product Master Data, Batch History, Blindly Reception, Report of Reception, Pre-dispatch Grouping by Client and by Product, Customer Invoice, Guide of Expedition to client.
Sensors: Thermo-hydrometer (instrument for measuring temperature and humidity), Data-Logger, GPS, Infrared pyrometers (temperature measurement instrument), and the Weighing Scales (weight measurement instrument).
Checkpoints: the verification of the packages weights, the entrance inspection in the Reception Area, the blindly reception in the warehouse, the verification of the number of units delivered in the warehouse, the verification of the batch and code of the product in the Dispatching Area, and the verification of the amount of packages dispatched and delivered in the Shipping area.
Coding system: The Uniform Product Classifier, CUP in its acronym in Spanish, is used in the company and throughout the drug chain of supply in Cuba. This code, erroneously used as an encoder, was replaced by the Cuban Product Classifier, CPCU in its acronym in Spanish, version 2.0 according to Resolution 49/2017 of the National Office of Statistics and Information, ONEI in its acronym in Spanish. The explanation of how this code is assigned is shown in Fig. 3, which shows that CECMED is involved in both the identification of domestic and imported products. In case of domestic, the decision of the code structure is associated with the regulations of GS1 Cuba, and those imported depend on the Issuing Agencies of codes in origin. This operation is not correctly implemented in the system, but instead an internal coding is used in each link, discarding the GTIN code.
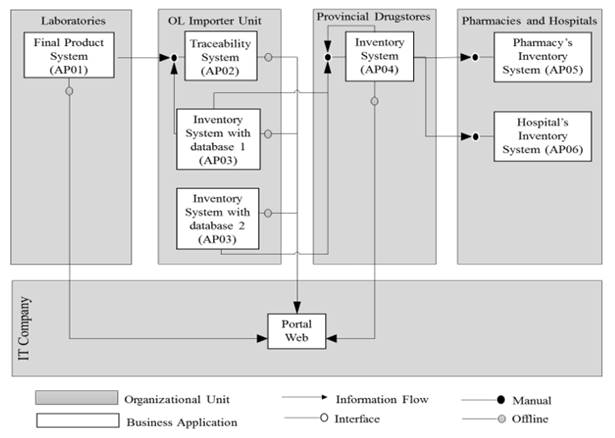
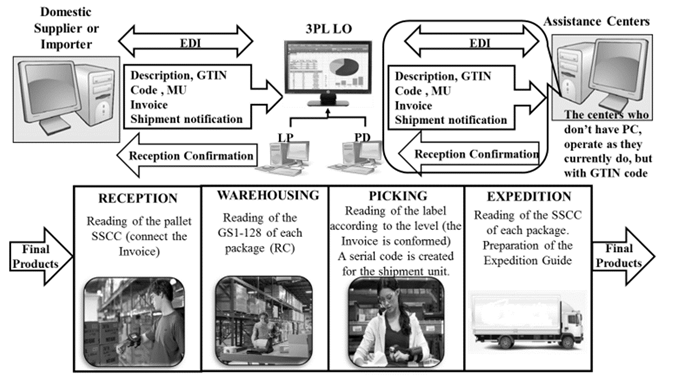
Computer systems: Fig. 4 shows the V-63 Pattern that illustrates the communication among the informatics systems of the different actors of the chain, which has a direct relationship with the traceability system of the drugs supply chain. The traceability information is introduced for the first time in the laboratories in case of a domestic drug, and/or in the LSP Importing Unit in case of an imported drug, and then, it is transmitted among these informatics systems. The drugstores can have access to a Web Portal, same as laboratories and some hospitals where information about the distribution and availability of drugs is shown, it captures the information of the existing informatics systems and allows to know, among other data and updated every 3 hours, the movements and records of dates, freighters data, place of reception (drugstore).

Source: The authors.
Figure 3 Example of CUP code assignation to a domestic product and an imported one.
3.3. Deficiencies in the traceability system
As a starting point of the investigation, different deficiencies were checked when detected in previous studies carried out between the Company and the University. However, the problems shown hereinafter constitute a deeper analysis of the traceability system, since they were detected from the realization of traceability exercises and monitoring activities through the informatics systems used in the supply chain, in addition to interviews made to Company specialists. In general, the traceability process in the drug supply chain in Cuba is carried out efficaciously, but it is very inefficient due to the poor use of standards and technologies for the fulfillment of the unique identification traceability principles, communication, data linkage, and recording and data capture [21].
Regarding the existent level of organization in the LSP, the deficient aspects found were:
Non-use of a product classifier controlled by the current legislation of the Country.
Non-use of an international standard product system for coding and identification.
The code of the manufacturer´s product is not sustained in the company.
The system for the products description does not guarantee their quick and unmistakable identification.
The units of measurement of products in the supply chain are not standard, for example, the unit of milligrams is used as mg or mgs one or the other.
The units of measurement of the products are not aligned to the International System of Units, SI in its acronym in Spanish.
Non-use of automatic identification systems in the company at the contact points with the client.
There are errors in the exchange of data among the different actors in the supply chain. The incorrect typing in the informatics systems of data, numbers of batches and origin of product, among others, are typical examples.
During the realization of the traceability exercises, various data could not be obtained, mainly because they were in paper format and were stored without an established filing system, this greatly hindered their search and non-satisfactory results were achieved.
Some data referred to the batches, such as the number of boxes, the box serial number and the location inside the warehouse, are not registered.
Regarding the level of technology in the LSP, the negative aspects found were:
The identification usually given to grouping of products that are dispatched is not registered in the informatics systems, only reflected in their packing and sometimes in the conformation work documents of these groupings (Pre-dispatch grouping by Client).
Each laboratory is identified differently in each drugstore.
The registration of data for traceability is not standard in the different drugstores, for example, in the smallest ones the exact date of entry of goods is not recorded, since they do not use the Registry Entry of merchandise to the warehouse, place where this data is required. It was also confirmed that sometimes all the information required by the records is partial or illegible.
In the reception and dispatching operations, data required to know the behavior of the physical movement is not registered in the informatics systems.
Virtually no product batch is linked with others that might have a coincidence on date and place during the travel among areas, in order to prevent cross contamination, even if for good practices, the manipulators know the products that cannot be close.
Problems of data veracity in the informatics systems were detected, because of records that do not match; for example, an entry date of a product to the drugstore appears anticipated regarding to the exit date of the Importing Company, where products come from.
The Web Portal does not illustrate the tracking of the products by batches, although the information is received from the Inventory systems.
There are problems of data veracity in the Web Portal such as repeated and incongruence information, for example, non-registered carrier data (Name and License plate of the vehicle) during the distribution of goods from the Importing Company to the drugstores.
The Web Portal does not specify any information in case of cross-docking from harbour to the drugstores. When this happens, the drugstore transmits by phone the required data to the LSP Import Unit who registers it in the Inventory System as an ownership transfer, which affects the necessary information for traceability and generates a false physical movement.
3.4. Implementation of improvements to the traceability system in the logistics system of drug distribution. Proposal of generalization.
One of the challenges faced today by Cuban companies in the health sector to increase their global competitiveness is to achieve the unique identification of each drug and its traceability through the supply chain, from the international or domestic provider to pharmacies, hospitals, institutes and other health care centers.
As previously analyzed, it is observed how the 3PL LSP of the drugs supply chain in Cuba is deficient for the achievement of an effective traceability. For this reason, it is proposed to implement improvements to this system based on the setting-up in a joint way of a Traceability Plan together with the use of the GS1 standards, a contribution in the generalization of this result.
3.4.1. Traceability plan
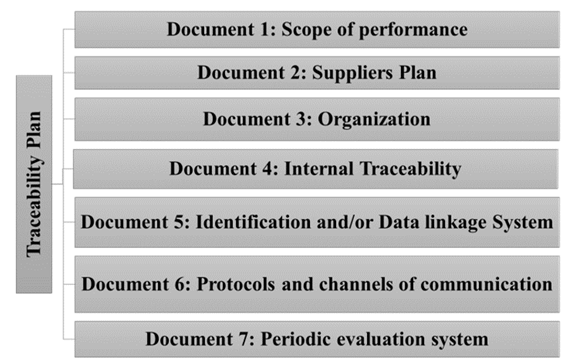
Based on previous studies of organizations and specialized publications, a Traceability Plan supported in important documents shown in Fig. 5 is proposed, with a reference stated in [22-26], creating the basis for basic notions of the Traceability System and its application by the internal staff and its understanding for any external personnel.
These documents must contain the following information [22-25]:
Document 1- The 3PL logistics Service Provider must define the scope of its Traceability System in both directions according to its commercial objectives, risk analysis or any other criterion. For this, it is also necessary to contact other actors in the supply chain and align their processes if necessary.
Document 2- The traceability plan must contain a provider plan establishing the necessary requirements (in terms of traceability) that suppliers must accomplish.
Document 3- This document must contain the description of staff and departments management responsibilities within the scope of the traceability system.
Document 4- This document should clearly include the descriptions of the handling flow and process, just like the description of the records and registration models.
Document 5- This document holds the traceability system and its technology. The features of the traceability informatics systems and their ways of using them should be described.
Document 6- This document describes the mechanisms to be activated to proceed to locate and withdraw from circulation of an affected batch with finished products.
Document 7- This document must establish the mechanisms to verify that the traceability plan effectively matches the proposed objectives, through the definition of the system evaluation process.
The information and/or procedures proposed in the Traceability Plan are within company documents, considering documents 2 and 6 the most advanced, while the others must be designed in an explicit way based on the proposed procedure for the implementation of GS1 standards.
3.4.2. Procedure for coding products using GS1 standards
The coding procedure to be used based on the GS1 standards was designed with reference to what stated in [21, 27-29] together with company's current procedures.
The success of the procedure will be even safer and larger in scope insofar as actors commitment and understanding increase concerning the need to achieve the unique identification of drugs in Cuba.
3.4.2.1. Principles for the application of the procedure
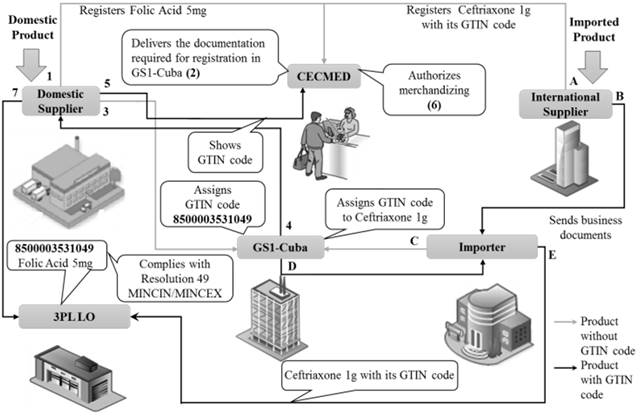
Carrying out the supply with quality, security and guarantee is not only a responsibility of the Logistics Operator, but from each actor in the supply chain, being that the integration of each one’s functions and acting as coordinated organizations, are the basis for efficiency in the logistics system of drug distribution in Cuba. That is the reason of demanding the GS1 standard in the supply chain from the highest regulatory authority in the country, as shown in Fig. 6:
The CECMED as a regulatory center of drugs merchandising in Cuba must ensure their identification by GTIN code.
The importer must demand in the contracts the products identification by GTIN code; otherwise it must be registered at the GS1 Office in Cuba´s Chamber of Commerce.
Domestic suppliers must register their products at the GS1 Office in Cuba´s Chamber of Commerce.
The relationships of CECMED with the rest of the organizations when the use of the GTIN code as a registration requirement is required would be as illustrated in Fig. 6.
3.4.2.2. Objectives of the procedure
General objective
To standardize the coding system in the logistics system of drugs distribution in Cuba.
Specific objectives
To achieve the unique identification of the products through the use of the GTIN code.
To require the identification of the products in commercial documents, packing and packaging.
To carry out the electronic exchange among the actors of the supply chain.
To computerize the data record of all operations.
To design the operation sub-processes: reception, storage, dispatch and expedition of the LO Importing Unit and the Provincial Drugstores.
3.4.2.4. Structure of the procedure
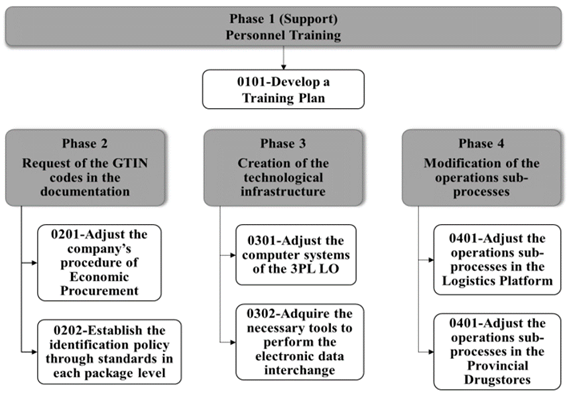
The coding procedure consist of four phases as shown in Fig. 7, each one with a set of steps indicating the activities to be performed.
3.4.2.5. First phase: personnel training
Any change in a company may generate resistance, processes redesign, increase of responsibilities, employee cutback and other consequences, which is why training of staff is essential. The plan to be carried out must be as detailed and organized as possible to guide both planning and teaching practices. The Training Plan of the company must establish: Scope, Purpose, Objectives, Goals, Strategies, Types, Pattern and Training Levels, Actions to be carried out, Topics to develop, Resources, Financing, Budget and Schedule.
3.4.2.6. Second phase: request of the GTIN codes in the documentation
The results expected at this phase are to achieve the obligatory identification of products with their proper GTIN code. This prevents recoding in other links of the supply chain.
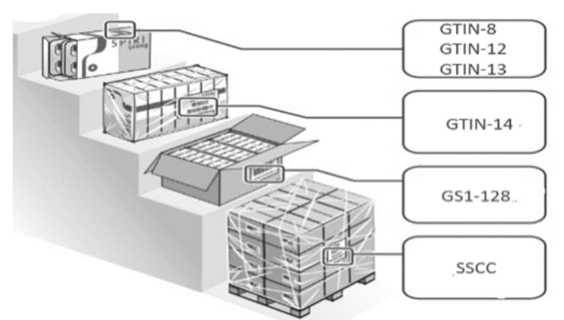
The Economic Contracting Procedure that rules the 3PL LSP contracts with domestic suppliers must be modified, in accordance with stablished legal provisions contained in clauses named Packing, packaging and marking of the goods, setting as an additional aspect the identification of products through the GTIN code. Penalties must be established in case that suppliers fail to comply with this clause. This clause must include, as shown in Fig. 8, the policy for the identification by the supplier in each packaging level, as well as the information obtained by the system from the codes. The LSP must also demand the submittal of the documentation that corresponds to the product before its reception.
3.4.2.7. Third phase: creation of the technological infrastructure
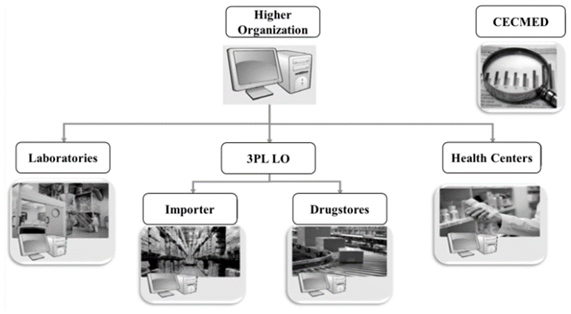
The company must create a technological infrastructure to support its processes to achieve the integrity of data, information exchange with other actors in the supply chain and access in real time. The system of each drugstore and platform must be managed from the Central Office. The database of the company and the remaining actors in the supply chain must be interconnected to a central base managed by the Superior Organization as shown in Fig. 9.
The introduction of the GTIN code in company’s operations involves installing a Wi-Fi network to guarantee the connectivity of portable terminals and modifying the information systems, mainly the informatics one. Currently this is not enough to progress, that is why a software for operational management in warehouses is needed. The LSP must adapt it for performing new functions and aiming to convert it into a Warehouse Management System (WMS), or then to acquire a software apart from the current system or to migrate to the use of an ERP system, which leads to other more drastic transformations. The transformations to be carried out are:
To allow automatic reading of data in the Reception, Storage, Dispatch and Expedition Areas.
To recognize the GTIN as the unique identification code of the product associated with the minimum presentation unit.
To assign the barcodes to the products, which will enter the system through Portable Terminals.
To allow the calculation of the verification digit in the internal use GTIN codes that identify the products without manufacturer's code.
To facilitate the electronic exchange of documents and the interrelation among the sent and received data using the same structure. Invoices issued by the supplier will enter the system without being typed by a user, decreasing the probability of error and increasing the process reliability.
The introduction of the information corresponding to the products in the database, their description, GTIN code and unit of measure, coming from the domestic supplier or the Importing Company, will be carried out in a centralized manner by an authorized personnel in those companies. The registered information of each product will be automatically displayed in the LSP Importing Unit and Provincial Drugstores through the electronic data interchange (EDI).
The EDI focuses on the transmission of information and supports the processes of the supply chain. It is used to transfer electronic documents or business data from one informatics system to another. In this way the staff of the different drugstores will not be able to introduce codes or descriptions, but the domestic suppliers and the importer the only ones responsible of ensuring the homogeneity through the supply chain.
The LSP, seizing these advantages, must begin to exchange documents beginning by:
Commercial invoices (Supplier - Drugstores, Importer - LSP Importing Unit, Drugstores - Clients).
Transfer among agencies (LSP Importing Unit - Provincial Drugstores).
New products registration (Supplier - Drugstores, Importer - LSP Import Unit, LSP Import Unit - Drugstores).
Reception confirmation.
Expedition Guides.
Claim Report (when applicable).
Tools required for the application of an automatic identification system in the LSP
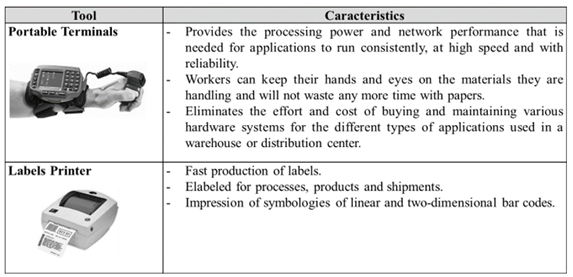
The application of this automatic identification system, in addition to the modifications to be done in informatics systems, requires a set of necessary tools for the execution of the processes. Table 1 shows the main technological devices to be acquired by the company with their characteristics and functions.
The acquisition of these tools and the installation of a Wi-Fi network by the LSP in all its units that guarantees the connectivity among the terminals is a significant investment for the entity. With the modifications of the informatics systems and the acquisition of these tools, the procedures that regulate the operations in the processes of reception, storage, dispatching and expedition must be modified.
3.4.2.8. Fourth phase: design of operation sub-processes at the 3PL LSP importing unit and provincial drugstores
In this phase, the sub-processes are modified as shown in Fig. 10, taking advantage of the opportunities offered by the unique identification of the products through the GTIN code.
4. Discussion
The efficient implementation of a traceability system in a 3PL LSP under the use of the GS1 standards, cannot be possible if all actors in the drug supply chain do not join this initiative, in this case in Cuba.
One result of the application in the study object has been knowing thoroughly by the health authorities in each country to see the exigency for using a unique identification of products as their direct responsibility in the logistics system. In American continent, it is outstanding the role played by the Food and Drug Administration (FDA) in the United States through rules and regulations contained in Federal Registries, likewise the National Sanitary Surveillance Agency (ANVISA in its acronym in Portuguese) in Brazil, and the National Drugs, Food and Medical Technology Administration (ANMAT in its acronym in Spanish) in Argentina. Then the requirement of the CECMED to national laboratories and the Importing Units of the Superior Organization and the Ministry of Public Health (MINSAP in its acronym in Spanish), which are the drug providers of the LSP about the use of the GS1 standard codes in the different packaging levels is necessary; for this, it is important to comply with the Joint Resolution MINCIN/MINCEX 1/2001, where it is required that any traded product in Cuba must be registered in the Chamber of Commerce, where a GTIN code by GS1-Cuba is assigned. The CECMED must watch over this when approving the merchandising of products, and then claim to be printed on the packaging.
In Cuba, the coding system to be used with the GS1 standards must be implemented together with the CPCU version 2.0 as required by Resolution 49/2017 of the ONEI and the harmonized system; this will enable the standardization of product information in the traceability system. In general, it is important to ensure that article identification systems must integrate the coding and classification systems in a joint and interconnected manner.
Despite the standards are the key to access all the information regarding the product to identify, only with the identification of the product in the packaging is not a guarantee for an optimum performance of 3PL LSP traceability system. The standardization of all data that support the information flow must also be established, such as the unequivocal identification of the actors in the supply chain, the previous definition of each one of the support processes and the necessary technological infrastructure for the capture and registration of the information. All these elements must be shaped into a Traceability Plan to understand the system, and to show its existence to any external entity that requires it to establish businesses. The Traceability Plans proposed in the literature have several formats and contents, but in this article, based on the experience acquired in the research, a structure is proposed, related to the standards of unique product identification, in this case those of GS1.
The above does not mean that with the existing technology improvements based on the GS1 standards in the 3PL LSP cannot be established to the traceability system. For the implementation of these improvements the fundamental thing is the standardization of the information, and the solution to the problems that informatics systems present.
The impossibility of using a technological infrastructure that supports the processes for capturing and recording data or that it is limited, conducts the traceability system to huge risks, that is why the company must perform the necessary analysis to assess the risks and benefits in different technological scenarios according to the capital sum willing to invest.
The implementation of a traceability system based on the GS1 standards provides improvements to the 3PL LSP logistics system such as:
Guarantee the security of health population and its consequent well-being.
Allow the efficient and economic evaluation of productivity and quality control.
Decrease in criminal acts.
Improves inventory management.
Control of logistics costs.
Control of logistics cycles.
The traceability system must be frequently evaluated as a part of the 3PL LSP internal controls, this guarantees its constant valuation and allows the continuous implementation of improvements.
It is important to highlight that the current traceability system in the analyzed logistics system is considered efficacious, but its fundamental weakness is the efficiency by which it is possible to carry out a TRACE or TRACKING process, which reduces its competitiveness when globally -:compared.
In the Traceability Plan and unique identification procedure the use of GS1 standards is proposed, as it is one of the 39 agencies that globally issues unique codes, complying with ISO/IEC 15459 version 2016-07-12, and in case of drugs as a final product its standards are the most used in the different levels of packaging.
The proposed traceability plan and its implementation through its procedure is considered generalizable to any drug logistics Service Provider. The use of the GLN (Global Location Number) for identifying each control point is also recommended, which along with automatic identification technologies with linear, two-dimensional or even RFID (Radio Frequency Identification) patterns allow the Geo-traceability process to be carried out, using qualified 4.0 industry technologies.
All the aforementioned entails not only investment in technology, but also a conviction of the logistics system actors with its consequent learning and training system to achieve the most important thing, which is patient safety and the resiliency of the drugs supply chains.
5. Conclusions
The diagnosis of the current traceability system of the LSP allowed to detect as fundamental deficiencies the lack of a unique identification for products in their transit through the supply chain and the non-use of international coding standards, the lack of computerization of some fundamental data to trace the products and incongruences in the data recorded in the Software used by the company.
Legal regulations in Cuba regarding identification through GS1 standards and traceability systems are behind existing international regulations in the health sector.
The proposed solution is centered on a traceability plan and a unique drugs coding procedure for distribution and trade under GS1 international standards. This proposal is considered liable to generalize to other Logistic Service Providers, regardless of the region where they operate.
The use of a traceability plan will allow the company to assess the traceability system from a more strategic view and have it documented as a basis for subsequent evaluations and improvements.
The application of the proposed procedure makes it possible to eliminate the detected deficiencies, achieving through the use of the GTIN code throughout the whole supply chain the unique identification of drugs in the Cuban health system and contributing to their traceability. The use of an international standard language facilitates the communication among the different actors in the supply chain, allowing them to communicate in the same language.