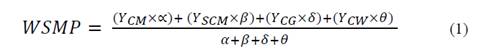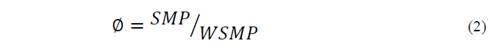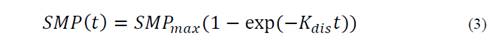1. Introduction
Anaerobic digestion (AD) is an effective technology for the conversion of organic wastes into methane-rich biogas and nutrient recovery [1]. In particular, chicken manure (CM) is an attractive substrate for anaerobic digestion owing to its high organic matter content, mainly comprising proteins [2]. Anaerobically, proteins hydrolyse into ammonia, which diffuses within microbial cells and disrupts cellular homeostasis. Ammonia concentrations over 2,500 mg/L reduce the methanogen population affecting methane yields [3].
The Anaerobic digestion of CM mixed with carbon-rich organic wastes has been proven to decrease the probabilities of ammonia inhibition and VFA accumulation [4]. The digestion of two or more substrates together known as anaerobic co-digestion (AcoD) can overcome several inherent problems associated with single substrate digestion such as the lack of micronutrients, imbalanced C/N ratio, and unfavourable (i.e. too high or too low) organic loading rates [5]. Abouelenien et al., (2014) [4] summarized the operational conditions for the codigestion of CM with agro wastes. In most of studies summarized in this report, mixture ratios were selected randomly to achieve optimal C/N ratios (25 to 30). However, mixtures with low C/N ratios have also been used successfully to achieve low partial increases in methane yield [6]. Moreover, the codigestion assays were conducted under wet conditions (solid concentrations of <10%), which is unfeasible for industrial application because of high water consumption and the too large required digester size [7]. Then, complementariness among physicochemical characteristics of wastes was not a decisive selection criterion to ensure the effectiveness of the mixture against further industrial application.
In AcoD, increased methane production is associated with both synergistic effects and an increase in organic load. Synergistic effects may include additional methane yield for codigestion over the weighted average of the individual substrate’s methane yield [8]. This may explain the increase in methane yield via the addition of the cosubstrate. Previous studies have reported stable digestion, enhanced gas yields, and improved economy of biogas plants in conjunction with this synergistic effect [9]. Biochemical methane potential and ammonia inhibition as well as synergy are directly linked to substrate composition [10]. The composition of substrate determines the efficacy of the microbial population, which in turn largely influences biogas yield, long-term process stability, and solid degradation rate [11].
On the other hand, methane production is largely influenced by the organic load or initial volatile solid (VS) concentration of the substrate in the digesters. The organic load and an accumulative volume of biogas are directly correlated [12]. If the organic load is very low, there is a risk of low microbial metabolic activity, in turn leading to low biogas production [13]. In contrast, if the substrate’s concentration is too high, the process may be inhibited by an overload of intermediate compounds such as VFA and ammonia [14]. Additionally, Mata-Alvarez et al. [15] documented that industrial AcoD plants should be limited by the transport cost of the cosubstrate from the generation point to plant location. In this sense, the increases in the organic loading rate will impact higher in the biogas plant cost-effectiveness rather than synergy.
Bec.ause of the potential for ammonia inhibition, biogas plants that treat chicken manure are forced to operate below full capacity. It is clear that the concept of synergy is still limited in its application to solving operational problems during the digestion process. Therefore, the impact of synergy on AcoD with high organic load concentrations must be determined in order to increase biogas production and reduce ammonia inhibition. Reliable AcoD modelling is needed to predict, in a clear and quantifiable manner, the effects of mixing two or more wastes in digesters and to mitigate any negative effects of this mixing [10]. Models are also useful in estimating important biochemical parameters, such as biodegradability, hydrolysis rate, and inhibition constant, which are critical in AD design, performance, and troubleshooting [10]. This study aimed to evaluate the synergistic effects of chicken manure with industrial wastes: sugar cane molasses (SCM), cheese whey (CW), and crude glycerol (CG) during AcoD. Cosubstrates were selected based on the ease to introduce them into the production chain of the Colombian poultry industry.
2. Materials and methods
2.1. Inoculum and wastes
The inoculum was cattle manure collected from a cattle slaughterhouse. The cattle manure was incubated at 25 °C to reduce residual organic matter content. The inoculum comprised a soluble chemical oxygen demand (COD) of 777 mg/L, 28.2 g total solid (TS)/kg, and 65% VS-to-TS ratio.
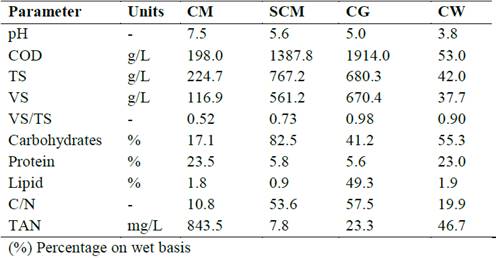
The substrate and cosubstrates were collected from Colombian industries. CM was obtained from a chicken farm, sugarcane molasses (SCM) from a sugar cane refinery, crude glycerol (CG) from an oil refinery and cheese whey (CW) from a dairy company. The characterization of wastes was shown in Table 2.
2.2. Identification of synergistic and antagonistic effects
2.2.1. Experimental set-up
The methanation assay was carried out in triplicate at 37±2 °C for 30 days according to procedures described by [16]. The initial VS ratio of inoculum to substrate was maintained at 2:1 throughout the experimental setup. Each 60-mL reactor contained an organic load of 9 g VS/L, 12 mL of inoculum, and sufficient distilled water to adjust total volume to 35 mL. Reactors were mixed by inverting once per day. Blanks containing inoculum and no substrate were used to correct for background methane potential in the inoculum. All reactors were purged with nitrogen gas and sealed using butyl rubber and an aluminium cap. Methane produced during methanation assay was quantified by the volumetric displacement of an alkaline solution. The volume of methane displaced was normalized and expressed in terms of specific methane production (SMP) m3 CH4/kg VS added.
2.2.2. Experimental mixture design
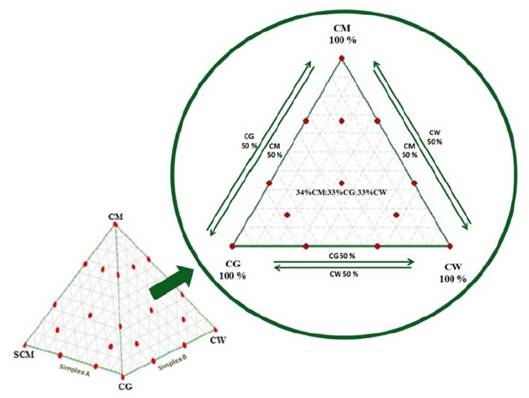
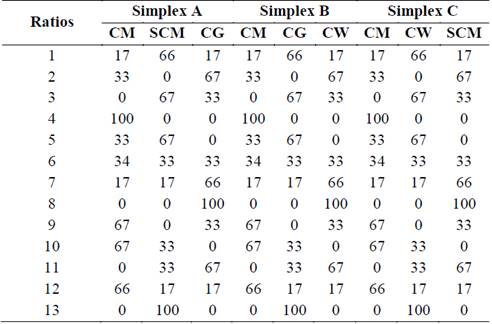
In order to eliminate the randomness of blending, the assay was run based on a simplex lattice design {4,3} augmented with three axial points. The mixture design was created using MINITAB 17 software (license 17.1.0.0) and represented graphically as a tetrahedron made up by a triangular base and three triangular faces called simplex (Fig. 1). Three simplex regions of interest were tested: A {CM, SCM, CG}, B {CM, CG, CW}, and C {CM, SCM, CW}. Each simplex consisted of 13 points (mixture ratios) where vertices corresponded to ratios with 100% single substrate. The upper vertex of the tetrahedron was the pure CM ratio which is also the upper vertex in each simplex. Vertices on the base of tetrahedron comprised pure ratios of 100% SCM, 100% CG, and 100% CW. Points on the axis corresponded to binary mixtures. Interiors points on each simplex corresponded to ternary mixtures. All points of the tetrahedron are listed in Table 1. MINITAB 17 was also used for statistical analysis of the experimental data via one-way ANOVA. Fisher least significant difference was calculated with 95% confidence to conduct pairwise comparisons of the SMP means.
2.2.3. Evaluation of synergistic and antagonistic effects
Synergistic or antagonistic effects were identified as a qualitative parameter for evaluation of process performance. Synergistic effects could be seen as additional SMP obtained during co-digestion over the weighted average of the individual feedstock’s SMP [8]. Weighted SMP (WSMP) was calculated using Eq. 1, as follows:
Where YCM refers to the SMP obtained from the digestion of CM as a mono-substrate. YSCM , YCG , and YCW are the SMPs obtained via singular digestion of their respective co-substrates. Moreover, / corresponds to the sum of the VS fractions added by CM, SCM, CG, and CW, in that order.
Synergistic effects were determined using Eq. 2, as follows:
Where SMP refers to the SMP achieved for the ratio tested. WSMP corresponds to the weighted average experimental SMP calculated using Eq. 1. If ∅ > 1, the mixture presented synergistic effects. If ∅ < 1, the mixture presented antagonistic effects. If ∅ = 1, the effects of the mixture during co-digestion were unclear.
2.3. Analysis of synergistic effects in terms of performance and inhibition
The ratios of each simplex with the highest synergistic effect were evaluated at organic loads of 9 and 18 g VS/L. The methanation assay was performed with the same operating conditions as in the previous stage. SMP and final TAN concentration were considered as response variables. The traditional first-order model was used to evaluate the kinetic degradation of the mixture with highest synergistic effects for both organic loads, according to Eq. 3:
Where SMP(t) refers to the SMY obtained with digestion time t, SMPmax refers to the highest theoretical SMP, and Kdis refers to the first-order hydrolysis constant. Model parameters were calculated using the curve-fitting toolbox (cftool) of MATLAB R2014a, license 271828.
2.4. Analytical techniques
Analyses of total solids (TS), volatile solids (VS), chemical oxygen demand (COD), total Kjeldahl nitrogen, total ammonia nitrogen (TAN), total organic carbon, proteins, and lipids were performed according to standard methods for the examination of wastewater of the American Public Health Association [17] The total amount of carbohydrates was estimated via Van Soest methods [18]. The pH values were determined using a pH meter (691, Metrohm).
3. Results and Discussion
3.1. Characterisation of wastes
The main characteristics of the substrate (CM) and cosubstrates (SCM, CG, and CW) are summarised in Table 2. All residues showed pH values below 6.5, except CM, with a pH value of 7.5. Moreover, residues had VS/TS ratios between 0.52 and 0.98, indicating high potentially biodegradable organic matter content. Organic matter in the co-substrates in particular was readily biodegradable, making the process susceptible to acidification. The SCM had the highest carbohydrate content at 82.5%, while the CG and CW had the highest lipid (49.3%) and protein (23.0%) contents, respectively. On the other hand, each residue presented a C/N ratio outside the optimal range (20 to 25) for the anaerobic degradation process [15]. The CM had the lowest C/N ratio, which is consistent with a high TAN of 843.5 mg/L. Nevertheless, the substrate and co-substrates provided conditions conducive to unprofitable anaerobic process.
Mixtures of residues could improve the biodegradability and stability of the anaerobic system.
3.2. Evaluation of synergy based on substrate composition
This section describes the synergistic effects of different carbohydrate, lipid, and protein concentrations during codigestion of CM with industrial agro wastes.
3.2.1. Specific methane production throughout simplex lattice design
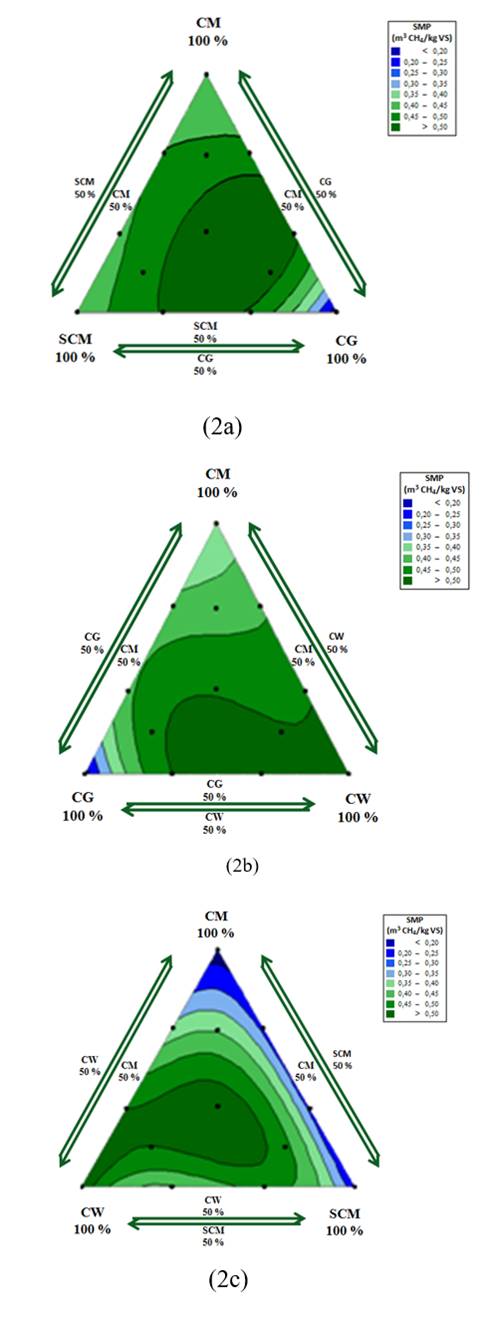
Fig. 2 shows the frontal view of each face of simplexes a, b, and c. Each view corresponds to a contour plot of specific methane production (SMP) created using MINITAB 17. These contour plots were useful in identifying the effects of cosubstrates that were rich in carbohydrates (SCM), lipids (CG), and protein (CW) during the co-digestion process with chicken manure. From the contour plots, the responses were analysed statistically to delimit significant regions. Each region was marked using a colour scale from blue to green according to the magnitude of the response. The darkest green areas had the statistically highest SMP, while deepest blue zones showed the statistically lowest SMP values.
Fig. 2a shows the effects of mixing chicken manure with cosubstrates rich in carbohydrates (SCM) and lipids (CG) in simplex A. The maximum SMP was obtained between the centroid point and the CM:SCM:CG ratio of 17:17:64. The average SMP obtained within this region was 0.45 ± 0.02 m3 CH4/kg VS, representing an increase of 32% over the SMP of CM alone. This finding indicates that SMP from the codigestion process was increased due to the increased lipid concentration in the codigestion mixture compared to that when CM was digested alone. The concentrations of CG producing the maximum response areas were 1.53 g VS/L and 5.94 g VS/L. These values are consistent with those recommended by the literature to achieve high rates of solids removal using glycerol as a cosubstrate [19]. Consequently, the simplex zone with lowest SMP corresponded to the right vertex (pure CG), where crude glycerol concentration was 9 g VS/L. Then, the right vertex presented high lipid concentrations in the digestion system with the possible overloading.
Fig. 2b shows the results of interactions between CM, CG, and CW in simplex B. The maximum response occurred in the area between the centroid point and the right vertex. For this region, the average SMP was 0.56 ± 0.03 m3 CH4/kg VS, equivalent to a 64% increase in SMP over that of CM alone. Then, affinity between CM and CW was presented for the simplex B. The affinity between CM and CW in particular could be a consequence of their protein-rich compositions. The affinity among the waste types reduced the lag phase increasing the biodegradability of the mixture [20]. Additionally, the high biochemical methane potential of CW facilitated an increase in SMP.
For simplex c, the greatest response was achieved at the centroid point, where the mixing ratio was 3 g VS/L for each residue (Fig. 2c). In this zone, the average SMP was 0.55 ± 0.04 m3 CH4/kg VS, equivalent to a 62% increase in SMP over that of CM alone. A lower SMP was achieved throughout the right adjacent area, where high concentrations of SCM were present. Moreover, the rapid biodegradation rate of carbohydrate-rich waste supports the elimination of latent phases in proportion with high concentrations of lipids and proteins [10]. Nevertheless, moderate supplementation of carbohydrate-rich residues such as SCM improved the biodegradability of lipid-rich residues such as CG and proteins-rich residues as CM.
3.2.2. Identification of synergistic and antagonistic effects
In AcoD, synergistic and antagonistic effects can be used as stability parameters for further dissection of the SMP data. Synergy mitigates ammonia inhibition and improves digestive process stability [9] and, therefore, SMP. Synergistic effects result from contributions of cosubstrates in terms of alkalinity, trace elements, nutrients, or any other features that the substrate itself is lacking. Similarly, antagonistic effects may be due to several factors, such as a drop in pH, ammonia toxicity, or high concentrations of volatile fatty acid [8].
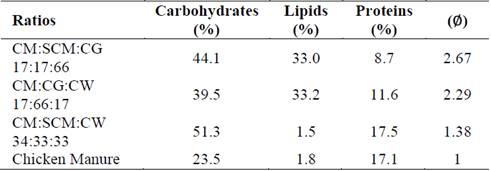
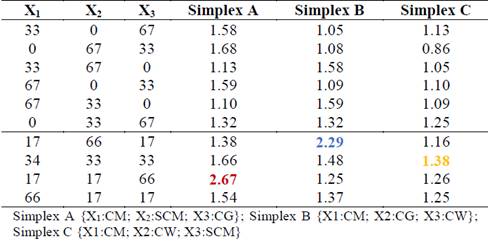
Table 3 shows the synergistic and antagonistic effects of mixtures of CM with residues rich in carbohydrates (SCM), lipids (CG), and proteins (CW). Synergistic effects occurred for binary mixtures when ∅ values were between 1.05 and 1.68. Most binary mixtures achieved synergistic effects, except for the CW:SCM ratio of 67:33, which presented an antagonistic ∅ value of 0.86. Antagonism reflects the instability of this mixture, which could have been due to the low pH values of CW and SCM. In general, all ternary mixtures produced synergistic effects, with ∅ values between 1.25 and 2.67, a higher range than that of the binary mixtures. The mixing ratios with the highest ∅ value in each simplex were considered optimal. For simplex A, the optimal synergistic ratio (∅ = 2.67) was the ternary mixture CM:SCM:CG of 17:17:66. For Simplex B, the optimal synergistic ratio was a CM:CG:CW mixture of 17:66:17, producing a ∅ value of 2.29. For Simplex C, the optimal synergistic ratio (∅ = 1.38) was the CM:SCM:CW mixture of 34:33:33. These findings confirmed the advantages of multi-component co-digestion over traditional digestion. [11] also found multi-component co-digestion to be a promising alternative in mitigating inhibition and improving anaerobic stability. According to their study, the synergy in the co-digestion of ternary and quaternary mixtures of solid wastes from dairy slaughterhouse plants (visors, blood, rumen) with manure, various crops, and municipal solid waste resulted in better distribution of nutrients, promoting rapid, beneficial development of microbial consortia.
Table 3 Synergistic and antagonistic effects (∅) of cosubstrates in binary and ternary mixtures with CM.
Source: The authors
Table 4 shows the nutritional composition (carbohydrates, lipids, and proteins) of the optimal synergistic mixtures. All optimal synergistic mixtures exhibited high carbohydrate concentrations, indicating that carbohydrates play a supporting role in anaerobic biodegradation process. Furthermore, proteins were found in intermediate concentrations for each optimal synergistic mixture, which could lead to controlled ammonia production in the anaerobic system. The CM:CG:SCM and CM:CG:CW optimal ratios of 17:17:66 and 17:66:17, respectively, had lipid concentrations reaching 33%. Such concentrations could be considered high when compared with those of the CM:CW:SCM optimal ratio of 34:33:33 and CM alone, which were below 1.8%. High lipid concentrations in mixtures seemed to improve ∅ values, possibly because of its large theoretical methane potential [21]. However, with high lipid concentrations in the digestion system there is a risk of overloading leading to VFA accumulation. Then, it could be concluded that Synergy could then be linked to the anaerobic system’s capability of sustaining a high lipid load during the digestion process. This could translate to hydraulic retention times for continuous optimization, resolving operational problems through synergy.
3.3. Operational outlook of synergy
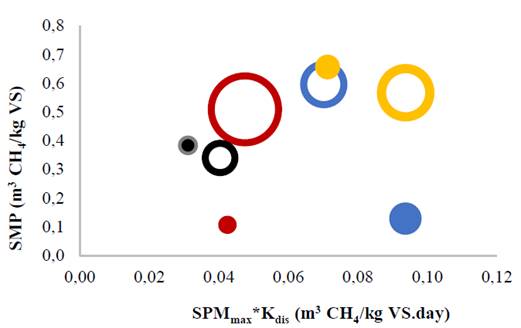
Synergy was assessed in terms of the impacts of kinetic parameters of biodegradability according to organic load. Fig. 3 shows a comparison between the SMP for optimal synergistic ratios and its uncertainty surfaces for kinetic parameters. Specifically, the maximum SMP and hydrolysis constant (Kdis) are the kinetic parameters that describe the substrate’s rate of biodegradation [22] (Galí et al., 2009). The size of the uncertainty areas corresponds to the standard deviation of the kinetic parameters. Filled areas correspond to the experiments with an organic load of 18 g VS/L, while the empty areas correspond to experiments with an organic load of 9 g VS/L. As shown in Fig. 3, the uncertainty of the kinetic parameters slightly increased with smaller organic loads, indicating that the substrate concentration must remain high to achieve reliable results.
Source: The authors
Figure 3 Comparison of uncertainty surfaces for methane production rate. Areas completely filled and empty represent organic loads of 18 and 9 g VS/L, respectively.
Fig. 3 also shows that the SMP for the CM:SCM:CW ratio of 34:33:33 significantly increased (p = 0.000) from 0.57 ± 0.02 to 0.66 ± 0.01 m3 CH4/kg VS via doubling of the organic load. On the other hand, SMP decreased with the CM:CG:SCM ratio of 17:17:66 and CM:CG:CW ratio of 17:66:17. For the organic load of 18 g VS/L, the CM:CG:SCM ratio of 17:17:66 and CM:CG:CW ratio of 17:66:17 achieved SMPs of 0.11 ± 0.01 and 0.13 ± 0.00 m3 CH4/kg VS, respectively. These values were lower than the SMP obtained with the control (CM monodigestion) of 0.38 ± 0.01 m3 CH4/ kg VS.
Via doubling of the organic loads, the CM:CG:SCM ratio of 17:17:66 and CM:CG:CW ratio of 17:66:17 increased Kdis to 0.38 ± 0.02 and 0.74 ± 0.02 days-1, respectively. For these loads, increases in the hydrolysis rate reduced SMP. High Kdis values indicate rapid hydrolysis rate of the soluble fractions of the residue [10]. This rapid hydrolysis rate is due to higher acidogenic activity, which is stimulated by increased nutrient availability with greater organic loads [13]. During digestion process, the cellular growth rate (Yx/s) of acidogens (0.15 to 0.17 g VS/g COD) is much higher than those of acetogens (0.025 to 0.051 g VS/g COD) and methanogenic archaea (0.020 to 0.054 g VS/g COD) [23]. Consequently, the anaerobic process requires organic loads with hydrolytic activity proportional to both acidogenic and methanogenic activity to avoid process instabilities. On the other hand, Kdis for the CM:SCM:CW ratio of 34:33:33 decreased from 0.15 ± 0.02 to 0.11 ± 0.01 days-1 when the organic load doubled from 9 to 18 g VS/L. The hydrolytic activity likely decreased proportionally with both methanogenic and acidogenic activity, maintaining cellular homeostasis. Kdis constants varied from 0.10 to 0.15 days-1, values similar to those commonly reported in the literature for chicken manure codigestion [7]. With an organic load of 9 g VS/L, Kdis values of the optimal synergistic mixtures were equal statistically to that of monodigestion. Codigestion with low organic loads therefore presented no significant benefit over the mono-substrate in terms of biodegradation rate.
In general, an organic load of 18 g VS/L resulted in antagonistic effects for the CM:CG:SCM ratio of 17:17:67 and CM:CG:CW ratio of 17:66:17. Meanwhile, the CM:SCM:CW ratio of 34:33:33 exhibited synergistic effects. Then, toxic compounds may have remained under the inhibition or saturation threshold for CM:SCM:CW ratio of 34:33:33. These results support a new approach for the evaluation of synergistic effects in AcoD. Synergy is effectively achieved with co-substrate supplementation when the organic load increases while negative factors such as pH, fatty acids accumulation, and ammonia toxicity are reduced during the digestion process. Effective synergy facilitates high biogas production rates and low hydraulic retention times, contributing to an optimal anaerobic system.
3.3. Inhibition outlook of synergy
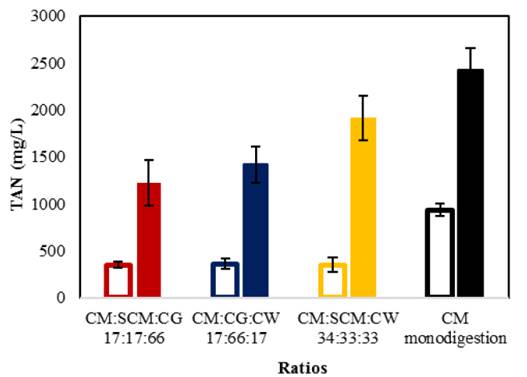
Fig. 4 shows the final TAN values for organic loads of 9 and 18 g VS/L. Monodigestion of CM achieved a final TAN concentration of 935 ± 66 mg/L, while the optimal synergistic ratios achieved TAN concentrations of 368 to 361 mg/L. With organic loads of 9 g VS/L, TAN concentrations for all assays remained below the inhibition threshold of 2,500 to 3,000 mg/L [3]. With organic loads of 18 g VS/L, the CM control achieved a TAN concentration nearly within the inhibition threshold at 2,427 ± 237 mg/L. Otherwise, TAN values of codigestion assays ranged from 1,220 to 1,912 mg/L, indicating that synergy between cosubstrates reduced the risk of ammonia inhibition. This can be explained by an optimum C/N ratio (25:1 and 30:1) Previous report show than CM codigestion present low concentration of TAN [24], which is consistent with the results of this study.
4. Conclusion
Synergy in the anaerobic codigestion of chicken manure with industrial wastes was stimulated with high concentrations of lipids in the mix. Synergy was then conditioned by tolerance and adaptability of the microbial consortia to a high lipid biodegradability rate. Organic loads below 9 g VS/L achieved synergistic effects because TAN concentrations ranged below saturation or inhibition thresholds. However, reliable synergy effects can be achieved to increasing organic load and methane production meanwhile negative impacts in the system are reduced. Multi-component codigestion is a viable strategy to achieve synergy, increase methane production, and reduce ammonia inhibition. As cosubstrates used in this study are easy introducible into the poultry value chain, anaerobic digesters could be implemented to improve the sustainability of the poultry industry.