1. Introduction
The last years, the investigations have been focused mainly toward the replace of materials derived from petrochemical resources for biodegradable materials with mechanical properties comparable to those of the no-biodegradable materials [1]. The biodegradable plastic materials offer a solution to the accumulation of synthetic plastic materials [2]. Starch is probably a more promissory material for the production of environmental friendly plastics among biodegradable polymers. This is due to its great abundance, low cost and easy of degradation [3]. Starch is a polysaccharide. Depending on the extraction sources, starch may have varying proportion of amylose and amylopectin, which confers to it several properties [3]. Furthermore, starch has a granular structure whereas thermoplastic polymer does not. Therefore, to obtain a thermoplastic starch (TPS), starch has to be plasticized [3]. The plasticizing agents are generally hydrophilic compounds, such as, urea [1], ethanolamine [4], glycerol [5], xylitol, sorbitol [6] and water [7].
During the plasticization process of the starch, the plasticizing agents form hydrogen bonds that decrease the interaction between starch macromolecules. Thus, there is a reduction of the granules sizes and increased mobility of the polymeric chains. Consequently, the processability and ductility are improved [7]. The starch proportion and chemical nature, strongly influenced the physical properties in two ways: 1) control the starch destructuration (it increases) and 2) affect the final properties of the materials such as, Tg and Young’s modulus [1,7].
A great deal of plasticizing agents has already been widely employed [4-7]. One material that may be employed as plasticizing agent to starch is the HBP of fourth generation. This material has been prepared from pentaeritritol and dimethylol propionic acid by polycondensation reaction by using a combination of the methods in a series of systematic approach [8-10]. This material has small hydrodynamic dimensions (3,28 nm) [8], an average number of terminal units of 9,36 (around 18 OH groups in the periphery) [9], lowest viscosity in molten state (lower than 2 x 101 Pa.s) [10] and its average number molar mass is 5030 g/mol [8].
There is little or no literature on HBP of fourth generation for this application. HBP have high structural packing, great OH groups number in the periphery, low viscosity in solution and molten state, lowest molecular entanglement and good solubility in commons organic solvents [11]. These materials may be a good alternative to the plastization of starch because their high OH groups number, which may interact and form hydrogen bonds with those of the starch. It may possibly reduce the interaction between OH groups of the starch and produce the destructuration of starch granules.
In a previous study, the use of a HBP of fourth generation in plasticizing of starch was reported [12]. The proportion of the HBP of fourth generation was 40 wt% relative to that of the starch (60 wt%). This material was named TPS60 [12]. The HBP was effective in the plastization of starch, since the crystallinity of starch was reduced. Furthermore, the thermal stability of the starch was higher than that of the TPS. In another study, was plasticized maize starch with hyperbranched polyesters, terminated with either hydroxyl (HBPET-H) or carboxyl groups (HBPET-C), these polyesters were prepared from glycerol and citric acid [13]. The plasticizing agents did not change the crystal structures of the starch films, but decreased the crystallinities. Also, both plasticizing agents increased the chain mobility. On the other hand, the HBPET-C was not as good plasticizing agent as HBPET-H. A hyperbranched poly(trimellitic glyceride) (PTG) was used on starch plasticization [14]. The tensile strength decreased with the PTG amount. Moreover, PTG did not change the crystal type of starch film, but reduced the crystallinities.
The effect of the proportion of a HBP of fourth generation on the starch properties has not been studied nor reported (according our literature review). Therefore, in this work, the influence of HBP amount on the structural, thermal, morphological, rheological and mechanical properties of TPS was evaluated. Furthermore, the properties of a TPS60 obtained in a previous study via the same methodology [12], will be compared with those of the materials prepared in the present study.
2. Experimental part
2.1. Materials
Tapioca starch was supplied by the Factory ALICO (Colombia), this material has a 17 wt% of amylose and 83 wt% of amylopectin. This material was previously dried at 50 °C in an oven for 12 h before being used. HBP was prepared by Murillo et al. and their properties have already been previously reported [8-10].
2.2. Preparation of the TPS
In order to prepare the TPS, the respective proportions of starch and HBP were carry out to the torque rheometer Thermo SCIENTIFIC and submitted to mixed process at 150 °C and a rate of 100 rpm during 8 min. The ratio of starch:HBP employed to prepare the TPS were: 30:70 (TPS30), 40:60 (TPS40) and 50:50 (TPS50).
2.3. Characterization of the materials
In order to characterize the materials, the TPS were kept in a desiccator before realization of IR, DRX, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and compression analyses. In the case of IR analysis, pills of the samples were prepared with KBr and were analyzed in a Perkin Elmer spectrometer model spectrum one. For every analysis 8 scans were done. In order to make DRX analysis, the samples were analyzed in a PANalytical X’Pert PRO MPD diffractometer by using Cu Kα= 1,54 A, the voltage and current operation were 45 kV y 40 mA respectively. The difractogramas were obtained in the interval of Bragg angle (2θ) of 4 and 70°. DSC analysis was performed in a TA Instruments Q-100 equipment in a temperature range between -80 and 150 °C by using a heating rate of 20 °C/min. TGA analysis was done in a TA instruments Q-500 equipment, the heating rate was 10 °C/ min. The DSC and TGA analyses were done in nitrogen atmosphere. SEM analysis was done to starch granules and cryofractured surfaces of the TPS, for it was used a JEOL JSM-6490LV microscopy. The samples were submitted in gold bath and the analysis were done using a voltage acceleration of beam 20 kV. Rheological analysis was performed in a Malvern Kinexus rotational rheometer by using plate-plate geometer of 20 mm of diameter at 130 °C under dynamic conditions in the range of angular frequency between 0,10 and 100 Hz and a strain of 0,2 %. Compression test was done only to compare the mechanical properties of the materials. Therefore, for it, were obtained cylindrical specimens of 3 cm of diameter and one cm of thickness. Compression test was performed in quadruplicate.
3. Results and Discussion
3.1. Torque rheometry
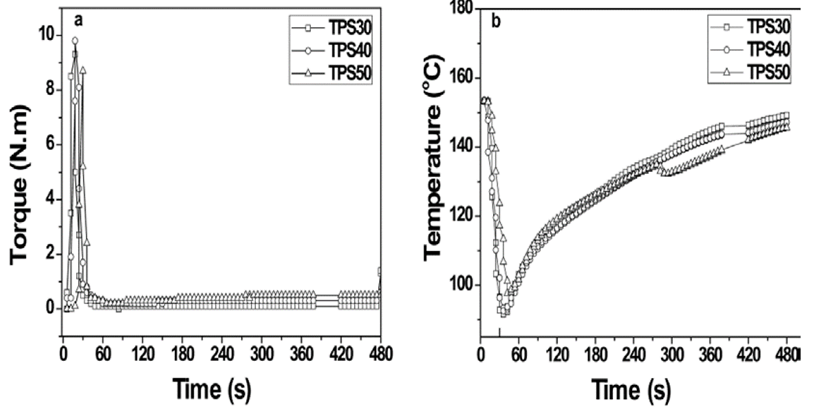
Fig. 1 presents the results of torque rheometry of the TPS. Initially, for all the samples, at 30 s of mixed process, the torque increased and then decreased and finally was stabilized (Fig. 1a). The samples exhibit a reduction in temperature (Fig. 1b). This may be because the melting process was endothermic and the increasing of torque (Fig. 1a) was produced for incomplete melting of the HBP. With the increasing of the HBP amount (melting temperature: 108,5 °C) [8], more heat for melting all the materials is required. On the other hand, the torque decreased with the enhancing of temperature, because in the same sense the HBP was melting. Finally, the torque reached a constant value at 280 s, this means that under conditions of mixed, the optimum time for obtaining the TPS (Fig. 1a) was established. The final torque value of the TPS, increased slightly with the proportion of starch employed. It was expected because the starch is not thermoplastic material. Therefore, the shear increased with the starch content. Possibly, the torque also is enhanced with the increasing of the interactions trough OH groups of HBP and starch. Once the torque was stabilized, no order reduction was observed, which indicates that, there was no degradation process during the processing. The same behavior was observed with TPS60 [12].
3.2. IR Analysis
In order to show evidence of interactions between starch and HBP the IR analysis was done. Fig. 2 presents IR spectra of the samples. Fig. 2a shows the IR spectra at high frequencies. The signal at 3399 cm-1 in starch is due to stretching of O-H bond of C-OH, which are bonded inter and intramolecular into starch structure. At 2940 cm-1 appear a signal corresponding to stretching of C-H bond of -CH2-.
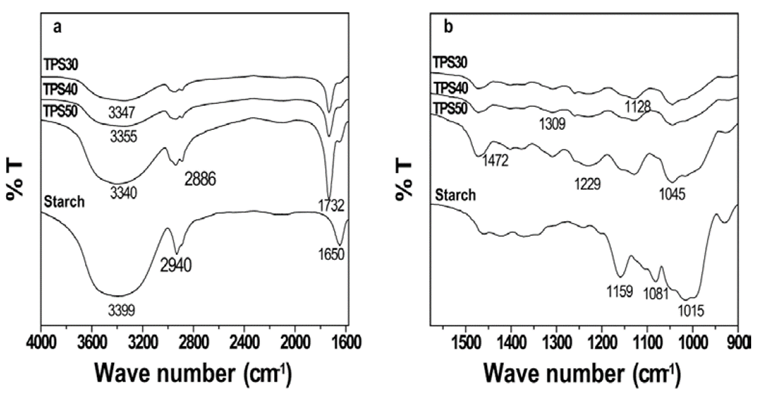
Source: Authors
Figure 2 IR spectra of the starch and TPS a) High frequencies and b) Low frequencies.
The signal observed in all TPS at 1309 cm-1 is assigned to bending of C-O bonds of the ester groups of HBP. This signal is absence in the starch spectrum. In the other hand, the signals at 1159 and 1081 cm-1 into IR spectrum of starch are attributed to C-O stretching of C-OH bonds [15]. These signals in the TPS IR spectra were observed to be displaced to lower frequencies (1128 and 1145 cm-1 respectively).
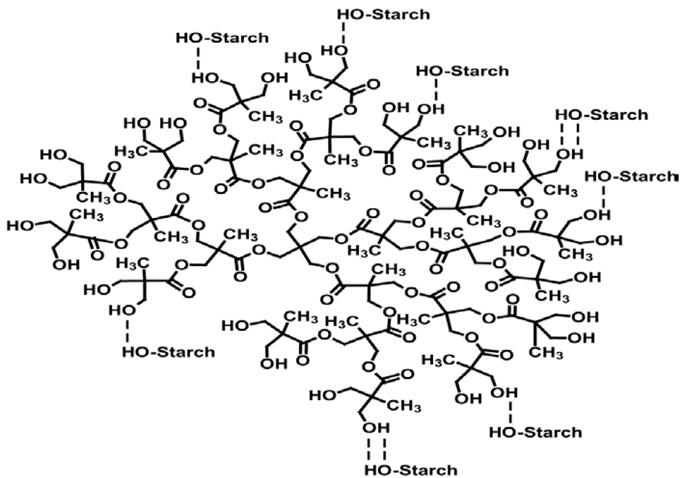
At 1015 cm-1 a signal which corresponds to stretching of C-O bond of C-O-C groups in the pyranose was observed [5]. This signal appeared into TPS spectra at 1010 cm-1 and its intensity was low with respect to that of starch. According to results obtained by IR analysis, it was evidenced that the presence of HBP in the TPS produced a displacement C-OH, C-O y C-O-C signals assigned to starch. This was attributed to the formation of hydrogen bonds between starch and HBP; which reduced the interactions between OH groups of starch. The displacement exhibited by these signals has been employed to show the evidence of the interactions between starch and plasticizing agents [1,4]. Therefore, all the samples exhibited interactions between starch and HBP. A schematic representation of these interactions can be observed in Fig. 3. The same behavior exhibited by the TPS in this study was also observed in TPS60 [12].
3.3. DRX analysis
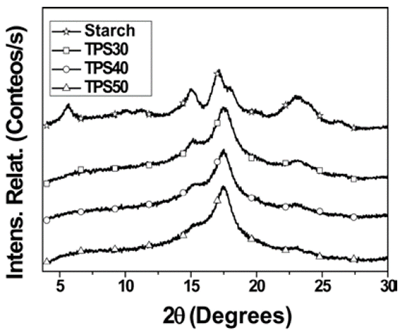
The difractograms of the sample can be seen in Fig. 4. Starch exhibited peaks of low intensity at 2θ=5,6o, 14.9o, 17,0o y 22,9o. Furthermore, three weak peaks at 2θ=9,8o, 11,4o and 26,5o were observed. The presence of peaks at 2θ=14,9o, 17,0o y 22,9o, indicate that the crystalline structure of the starch is mainly A type, but also there is a B type crystalline structure (2θ=5,6°) [16]. On the other hand, the C type crystallinity is considered a mix of crystallinities A and B types, which exhibit the same diffraction patron of an A type crystal with an additional peak around 2θ=5.0o, which is characteristic of B type crystallinity [16].
The TPS difractograms did not exhibit the peaks at 2θ=5,6o, 9,8o and 11,4o and the peaks at 2θ= 14,9° and 22,9°, ostensibly reduced their intensity. The reduction of the intensity of these peaks and the absence of other can be interpreted as a reduction on crystallinity of starch in TPS. Although the peak in the TPS difractograms at 2θ=17° increased slightly its intensity with regards to that of the neat starch.
The peaks at 2θ=14.9° and 22,9° enhanced in intensity with the HBP content, which can be interpreted as an increasing of the A type crystallinity in these samples. This may be attributed to those samples with high proportion of HBP. The shear is low due to the reduction on viscosity and it reduces the destructuration of starch, thus, it can be corroborate from results obtained by rheometry, since final torque increased with the proportion of starch (Fig. 1). The TPS50 was the sample that showed the lowest reduction on crystallinity.
The TPS60 exhibited the same peaks of the TPS [12], but in the case of the peaks at 2θ=14,9° and 22,9°, their intensities were slightly higher than those of the TPS obtained in this study. This behavior may be attributed to high proportion of starch. Possibly this sample exhibited high resistance to the mixed (was obtained with highest proportion of starch) and different interaction or destructuration degrees.
In this study, there was no evidence of the formation of V type crystallinity, since it was not observed in new peaks, as in the case of the other plasticizing agents such as, glycerol, sorbitol and ethylenglycol [17]. This indicates that HBP is more efficient in restrict the formation of this type of crystallinity. The absence of this type of crystallinity in TPS was probably due to the interaction between OH groups of the amylose and HBP, which possibly produced the stabilization of amylose helix.
According to the results, it can be concluded that the HBP acts as plasticizing agent of the starch, since HBP reduced the crystallinity of starch. The efficiency of HBP possibly was due to this material exhibiting high structural packing, low viscosity in molten state and high OH groups number OH [8-10].
In this study, was not observed the recrystallization (retrogradation) for the TPS, which was due to the interaction between starch and HBP, since it reduced the hydrogen bonds between the starch chains [18].
According to results obtained by DRX, A type crystallinity was eliminated by HBP and the B type crystallinity of starch was reduced. In a study of the starch plastization by using HBPET-H, HBPET-C and PTG, it was observed that these plasticizing agents did not change the crystal type of starch, but they decreased the crystallinities [13,14].
3.4. Thermal analysis
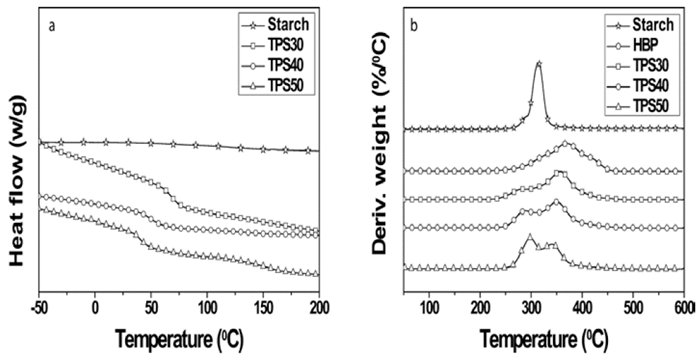
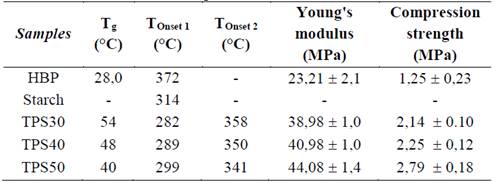
The DSC (Fig. 5a) and TGA (Fig. 5b) thermograms of the samples are displayed in Fig. 5. In DSC thermograms(Fig. 5a) it can be seen that starch did not exhibit a Tg. All TPS presented only a Tg value and it was higher than that of the HBP (28 °C) [8]. The absence of Tg of HBP in these samples is another indication that there was interaction between starch and HBP. Furthermore, in previous study it was observed that a small endothermic peak to HBP was assigned to the melting of ordered domains of hydrogen bonds due to the interaction of terminal OH groups [8]. This peak was not detected to TPS, which means that the HBP is interacting through OH groups with starch. All Tg values of the TPS are presented in Table 1. The Tg values increased with the HBP amount. The sample TPS50 exhibited the lowest Tg value (40 °C), which means that this sample showed highest destructuration of the starch granules and dissociation of interactions between OH groups of starch. Therefore, the mobility of the starch chains was consequently increased. [19]. The sample TPS60 presented a Tg value of 31 °C [12], which indicate that this sample has a higher interaction between starch and HBP than the TPS obtained in this study. An explanation for the behavior showed by TPS and TPS60 is that when the content of HBP is higher than 50 wt% (TPS30 and TPS40), the starch granules are more disperses into the HBP and as consequence of the shear, these were separated, but their interaction with the HBP was lowest, which probably was due to low viscosity. Therefore, the interactions between HBP and starch are lowest for these samples, because probably there is great piece of HBP without interact with starch. In another account, plasticized corn starch with glycerol (15 wt%) and urea (15 wt%), el Tg value was 37 °C [1], this value is comparable with the TPS50 and TPS60. Kaur et al. [20] obtained a Tg value of 63 °C for plasticized potato starch with glycerol monoestearate and water (1:10).
In the TGA thermograms of the samples (Fig. 5b). The starch and HBP exhibited only one peak, the thermal stability of the starch is higher than that of the HBP. The TPS presented two peaks, the first peak (Tonset1) increased your intensity and your thermal stability (TPS30→TPS50) with the enhancing of the starch amount, but its thermal stability was lower than that of the starch. These results indicate that this peak is related to starch. Furthermore, the increase in thermal stability of this peak may be associated with enhancing of the interactions between starch and HBP. On the other hand, the reason for the lowest thermal stability of this peak with regards to that of starch is probably attributed to the interactions into starch are more strong than those into TPS. The second peak (Tonset2) decreased its intensity and thermal stability with the reduction of HBP amount (Fig.6b and Table 1), which indicate that this peak is associated to HBP present into the TPS. The same behavior was observed to TPS60 whose values of Tonset1 and Tonset2 were 302 °C and 339 °C, respectively [21].
The displacement of the Tonset of starch and HBP in the TPS is a proof that the interaction between starch and HBP was carried out and it decreased with the proportion of HBP. The presence of peaks due to starch and plasticizing agents has been reported in the plasticization of starch with glycerol [22].
3.5. Rheological analysis
Fig. 6 shows the rheological behavior of the samples. The result of dynamic analysis (Fig. 6a) shows that all samples exhibited a reduction on complex viscosity ((*), in the case of HBP it was due to dissociation of interactions through OH groups, which has been seen in HBP [11]. On the other hand, the reduction on viscosity of TPS probably corresponded to dissociation of interactions and disentanglement of starch chains (amylose or amylopectin). The (* values of the samples at angular frequency of 1 Hz are as follow: HBP: 16,89 Pa.s, TPS30:16,99 Pa.s, TPS40:30,39 Pa.s and TPS50:58,03 Pa.s. Accordingly, this result means that the order of the interactactions degree is as follows: TPS50(TPS40(TPS30. Furthermore, this result followed the same trend exhibited by these samples on final torque. In addition, the TPS50 presented a lowest reduction on (*, this means that this sample exhibited a highest stability under conditions used to make this analysis; this is corroborate by TGA results. Furthermore, this sample showed a great displacement of the Tonset1 and Tonset2 regards to Tonset1 of the starch and HBP, which was due to highest interaction between these materials. TPS60 exhibited a (* at 1 Hz of 486,99 Pa.s [12], this value is highest relative to that of the TPS. This may be due to high number of interactions and the starch content. On the other hand, at an angular frequency of 20 Hz, it was observed that a considerable increase on (* value for all TPS (shear thickening), the same was observed with TPS60 [12]. This can be interpreted as a formation of a microstructure, which is the ability of elastically deformation when exposed to external stress. This behavior has already been detected for tapioca starch dispersions [23]. The formation of this microstructure has been assigned to complexation reaction between amylose and lipids [24]. The angular frequency value, at which the increasing abrupt appear on (* did not showed a trend with the starch and HBP proportions. The rheological behavior exhibit by the HBP and TPS (Fig. 6b) was mainly viscous because loss modulus (G'') was higher than elastic modulus (G'). The considerable increase on G'' and G', was due to the microstructure formed, but did not occur a transition from viscous to elastic (G'' ( G'), which has been observed in starch gels [25]. The (* values of the samples at angular frequency of 1 Hz are as follow: HBP: 16,89 Pa.s, TPS30:16,99 Pa.s, TPS40:30,39 Pa.s and TPS50:58,03 Pa.s. This result followed the same trend exhibited by these samples on final torque. In addition, the sample TPS50 presented a lowest reduction on (*, this means that this sample exhibited a highest stability under conditions used to make this analysis; this is corroborate by TGA results. Furthermore, this sample showed a great displacement of the Tonset1 and Tonset2 regards to Tonset1 of the starch and HBP, which was due to highest interaction between these materials. TPS60 exhibited a (* at 1 Hz of 486.99 Pa.s, this value is highest relative to that of the TPS. This may be due to high number of interactions and the starch content. On the other hand, at an angular frequency of 20 Hz, it was observed that a considerable increase on (* value for all TPS (shear thickening), the same was observed for TPS60 [12].
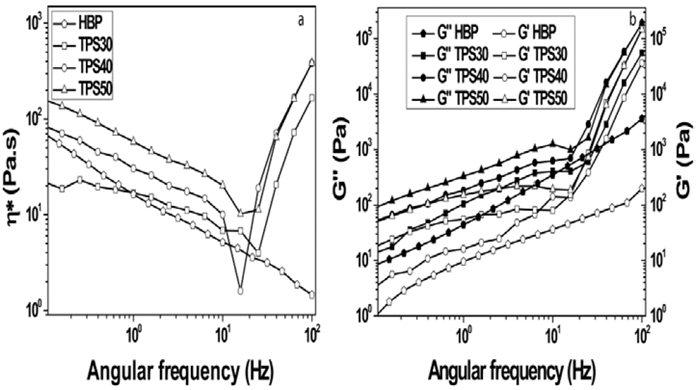
Source: Authors
Figure 6 Rheological behavior of the HBP and TPS a) (* vs angular frequency and b) G'' and G' vs angular frequency.
This can be interpreted as a formation of a microstructure, which is the ability of elastically deformation when exposed to external stress. This behavior has already been detected for tapioca starch dispersions [23]. The formation of this microstructure has been assigned to complexation reaction between amylose and lipids [24]. The angular frequency value, at which the increasing abrupt appear on (* did not showed a trend with the starch and HBP proportions. This means that the size of these microstructures and the interactions are different.
The rheological behavior exhibit by the HBP and TPS (Fig. 6b) was mainly viscous because loss modulus (G'') was higher than elastic modulus (G'). The considerable increase on G'' and G', was due to the microstructure formed, but did not occur a transition from viscous to elastic (G'' < G'), which has been observed in starch gels [25]. These results indicate that the gel content or microstructures present in TPS is lowest.
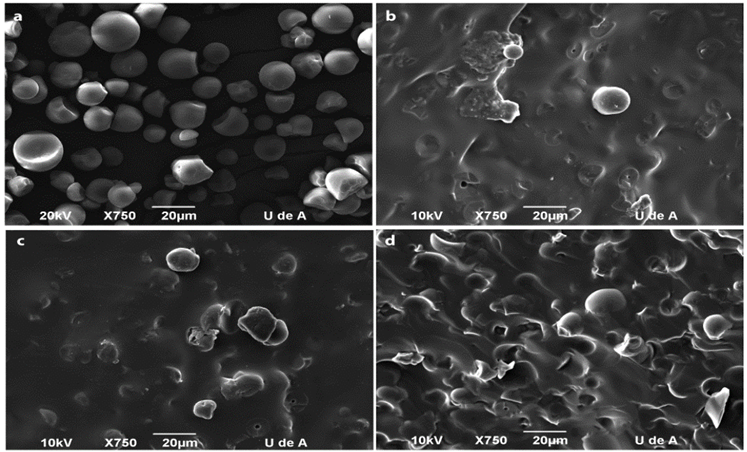
3.6. SEM analysis
The morphology of the samples is presented in Fig. 7. It can be observed that the starch granules sized into TPS are smaller than those of the neat starch. That was as a result of destructuration produced by shear and HBP. In addition, similar results were found for starch plasticized with a mix of urea-ethanolamine [1]. In a study of the plasticization of starch with glycerol was reported that the addition of polyol to starch matrix changed the microstructural arrangement of starch chains and are less dense [26,27]. The samples TPS30 and TPS40 exhibited in some regions without starch granules. Big domains without starch particles were observed in TPS50, which indicates that this sample has a greatest interconnection. This behavior is possibly due to the increasing of shear during the processing since this sample was obtained with the highest amount of starch. TPS60 [12] displayed the same behavior to that of TPS50. The results obtained in this study corroborated the results found by DSC analysis, since it was observed that TPS50 showed better interaction between HBP and starch than TPS30 and TPS40. The results obtained by SEM confirmed the reduction on crystallinity observed with the samples TPS30 and TPS40. This was possibly due to high dispersion of starch due to smallest starch amount present in these samples and not the high interaction between starch and HBP. Furthermore, it is possible that the effect of HBP in these samples be mainly as a result of dispersant agent present in these proportions. HBP is the dispersant phase.
3.7. Compression test of the samples
The Young’s modulus and tensile strength of the TPS decreased with the HBP content and these were higher than those of the HBP (Table 1). This is possibly due to the highest interaction between HBP and starch and to the presence of starch in the TPS. The results of Young’s modulus, are lower than those reported to starch plasticized with ethylene glycol, propylene glycol and sorbitol (10-40 wt%), whose values were between 59 and 219 MPa [17]. According to the results, TPS did not improve the mechanical properties of the TPS, probably because the material is fragile.
4. Conclusions
In this study, TPS was obtained by employing a HBP as plasticizing agent. All the TPS present an interaction between starch and HBP. The proportion of HBP affect the shear degree of the materials during the processing, because the TPS prepared with lowest proportion of HBP, exhibited the best interaction between starch and HBP. This was due to highest viscosity of the system. Therefore, it favored the destructuration of the starch granules. The crystallinity of the TPS was lower than that of the starch and it increased with the proportion of HBP. None of the TPS exhibited V type crystallinity, which indicate that HBP restrict this crystallinity type, it can be interpreted as a high stability against retrogradation of starch, which is an important result. The rheological behavior of the TPS in the range of angular frequency studied was manly viscous. Furthermore, all TPS exhibited the formation of a microstructure. On the other hand, the size of the starch granules decreased comparably to the neat starch. The increase of HBP amount reduced the viscosity and mechanical properties of the TPS. However, the HBP may be an alternative for plastization of starch. According to the results, HBP affect the structural, thermal, morphological, rheological and mechanical properties of the TPS, since this material interacted with starch.