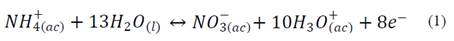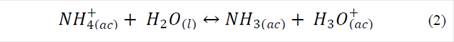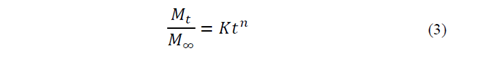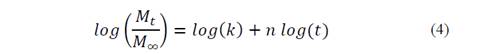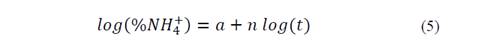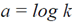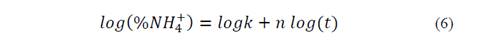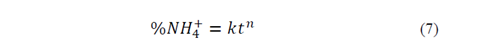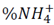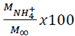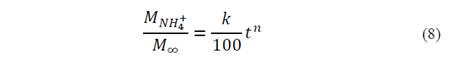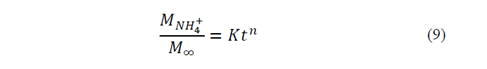1. Introduction
Nitrogen deficiency in soil limits the yield and quality of produce [1]. Soil matrices can lose nitrogen via volatilization, lixiviation and denitrification processes; these affect soil recovery and fertilizer performance [2] -they also generate pollution [3]. Thus, researchers have tried different approaches to reduce N exit from soil: choosing the right source of N, adjusting the dose of fertilizer and setting the correct time and method of application.
Controlled-release fertilizers are gathering momentum due to their ability to prevent nutrient loss [4]. Researchers have recognized their capacity to minimize environmental impact [5,6]. Still, controlled-release fertilizers remain in a stage of development and require further research to minimize production costs -in that way, a trend toward waste-based fertilizers has surfaced over the past few years [7].
The pulp and paper industry, one of the largest contributors to pollution, generates large amounts of waste that constitute an environmental puzzle. Recycling of this waste produces enormous environmental benefits, but so far, researchers have only used it as material for construction[8]. We expect this material could work to produce new fertilizers, but although paper sludge waste is widely available, we have not found any prior research stating that.
Some authors have used raw materials derived from agribusiness waste, and they highlight the importance of adjusting preparation parameters to match the nutritional requirements of the plants [7]. Other authors have added materials such as Bentonite clay and copolymers to waste-based fertilizers; these improve mechanical strength and release performance, which translates into a smaller nutrient loss [9]. Although these authors have demonstrated that changes to the composition of the device can improve its performance, the effect of the physicochemical parameters of different matrices on that performance remains unknown.
In this research, we developed a residual cellulose/polyvinyl alcohol controlled-release fertilizer. We then used the Total Kjeldahl Nitrogen method to determine the amount of ammoniacal nitrogen lost from soil and sand matrices and finally, assessed the effect of the physicochemical properties of these matrices on nutrient release.
2. Materials and methods
2.2. Reagents
Analytical grade sulfuric acid (98%), hydrogen peroxide (50%), Nessler polyvinyl alcohol, Kjeldahl Nitrogen indicator and 12N potassium hydroxide were obtained from Hach. Lactophenol blue was obtained from Merck. Glucose Sabouraud agar (4%) was obtained from Becton Dickinson GmbH. Diammonium phosphate (DAP) with a composition of 18% nitrogen, 46% phosphorus and 0% potassium was obtained from Delcorp.
2.3. Materials for planting
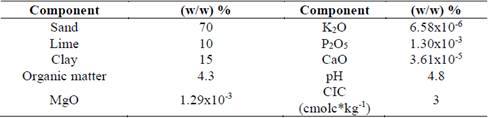
Residual cellulose/polyvinyl alcohol hydrogel was manufactured at Universidad EIA (Table 1). Sieved and washed sand (2 mm) and Andisol (Fulvudand), were provided by SOBIOTECH (Table 2) and characterized by Grupo Interdisciplinario de Estudios Moleculares-GIEM at Universidad de Antioquia and Laboratorio de Suelos at Universidad Nacional de Colombia, in Medellin, respectively. Zea mays seeds (Poaceae) 96 % pure and 80 % Germ were kept away from light, at a relative humidity of 30 % and a temperature of 4 °C, to inhibit germination. The seeds’ surfaces were disinfected with water and 70 % alcohol for 2 min. Then, they were immersed in 0.05 % sodium hypochlorite for 5 min, under constant stirring. After disinfection, the seeds were washed with sterile water and laid over wet sterile paper sheets for two days to complete germination. Germinated seeds were placed in plastic cylindrical holders (11 cm x 9 cm) with 200 g andisol type soil and thinned after 15 days to limit the number of plants to one per holder.
Table 1 Physicochemical analysis of residual cellulose/polyvinyl alcohol, data in (m/m) %. RC = Residual Cellulose, PVA = polyvinyl alcohol.
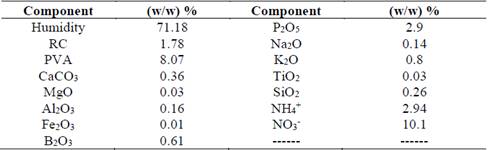
Source: The authors
2.4. Equipment
DR/200 UV/Vis spectrophotometer (Hach). 400-900nm. Tungsten halogen lamp. Spectral band: 7nm. Wavelength: ± 2 nm. Resolution: 1nm. Photometric linearity: ± 0.002 A. Photometric reproducibility: ± 0.005 A.
Digesdahl Digestor (Hach). Model 23130-20. Temperature range: 100 °C - 400 °C. Temperature control ± 15 °C. Flushing capacity: 11.5 L/min at a water flow rate of 6.5 L/min. Minimum pressure: 51.7 kPa.
Phenom Scanning electron microscope. Optical zoom: 20x -135x. SEM zoom 80x - 130000x. Sample size 32 nm. Acceleration voltage: 5 kV - 10 kV.
Olympus optical microscope CX21. 10x/18 ocular. Binocular tube inclined at 30°. Interpupilar distance range: 45 mm - 75 mm. Rotatable quadruple lense holder. 4x, 10x, 40x and 100x lenses. Halogen lamp 6V/20W. Fixed mechanical slide with cable movement: 120 x 132mm. Range of displacement: 76 mm(X) by 30 mm(Y).
2.5. Methods
2.5.1. Nitrogen quantification
Solid sample treatment: 0.1 g to 0.5 g of residual cellulose/polyvinyl alcohol hydrogel were crushed with mortar and pestle and then placed in a 100 mL Digesdahl® flask alongside 25 mL of distilled water. Then, 3 mL of 98 % sulfuric acid were added into the flask and stirred. 10 min later, the flask was placed in a digester and heated at 440 °C. Once reflux started, the flask was allowed to set for 16 min, and then, 10 mL of 50 % hydrogen peroxide were added through the capillary funnel. Once hydrogen peroxide was added, heating was suspended, and the flask was again left to set for 30min. The digested solution was filtered through a 595 Rundfilter paper (( 110 mm) and taken to a 100 mL volumetric flask, where the final volume of the mixture was adjusted using distilled water.
Liquid sample treatment: 25 mL of the leachate were taken to a 100 mL Digesdahl® flask, and the same method used in the treatment of the solid sample was followed.
For the determination of ammoniacal nitrogen as Total Kjeldahl Nitrogen by Nessler 399 method, Hach 0875 method was used: 3 mL of the prepared solution were taken to a 25 mL volumetric flask, and then, a drop of TKN indicator was added into the flask, alongside three drops of mineral stabilizer, three drops of a dispersing agent (polyvinyl alcohol), 200 uL of 12 N potassium hydroxide and 1mL of Nessler's reagent. Distilled water was added until a 25 mL solution was obtained. The solution was then taken to the spectrophotometer.
2.5.2. Evaluation of the hydrogel’s degradation
The hydrogel was cut in 1 cm x 1cm pieces. The pieces were dried at 20 °C for 48 h and weighed. Then, they were placed in distilled water (pH 6.8) for 30 days, and dried every five days at 20 °C for 48 h and weighed again. All tests were run in triplicate.
2.5.3. Assembly for nitrogen release leaching tests
Polyvinyl chloride (PVC) cylindrical columns (20 cm long by 3.8 cm wide) were adapted with a nylon filter at the lower end. Each column was packed with 200g of the substrate (andisol or sand) and 525 mg of the hydrogel. The columns were washed using 120 mL of distilled water, and then, the leachate was collected for analysis; this process was performed on days 3, 5, 15, 25, 30 and 60. All tests were run in triplicate. 194 g of diammonium phosphate were used as positive control [10]. Presence of nitrogen was determined by spectrophotometry using the Nessler 399 method.
2.5.4. Assembly for greenhouse tests
Hydrogel and diammonium phosphate were added to the soils where Zea mays seeds had previously germinated. The plants were grown in natural light in SOBIOTECH’s greenhouse in Antioquia (6°15´07" N; 75°25´42" W, 2117 masl). The soil was kept around 50 % to 60 % of its maximum moisture retention capacity. The plants were harvested 60 days after planting, their height was measured, and then, they were dried at 50 °C for 48 h to determine their total dry mass. All tests were quintupled.
2.5.5. Microbiological analysis of soil
Microbiological analysis of the soil used in the greenhouse tests was performed to determine the presence of mold, yeast, or bacteria before and after 60 days of treatment. A pattern solution was obtained by mixing 10 g of soil and 90 mL of distilled water. Serial dilutions of this solution were obtained by mixing 1 mL of the solution and 9 mL of distilled water until a concentration in the order of 10-7 was obtained. 100 uL solutions of concentrations 1x10-1, 1x10-3, 1x10-5 and 1x10-7 g/L were placed in Sabouraud Agar.
3. Results and discussion
3.1. Degradation of the hydrogel
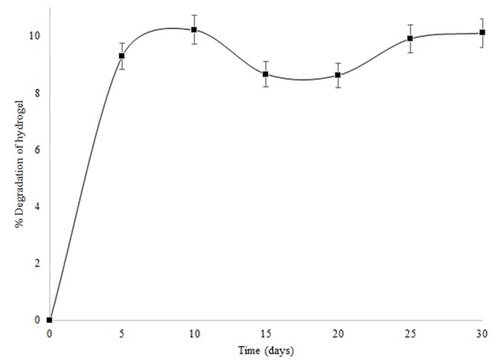
Fig. 1 shows that, from day five on, hydrogel loses up to 10% of its structure. This reduction in mass is related to the erosion of residual cellulose and polyvinyl alcohol chains that failed to crosslink during hydrogel formation; this behavior indicates low disintegration during the evaluation period.
3.2. Release of NH 4+ by leaching
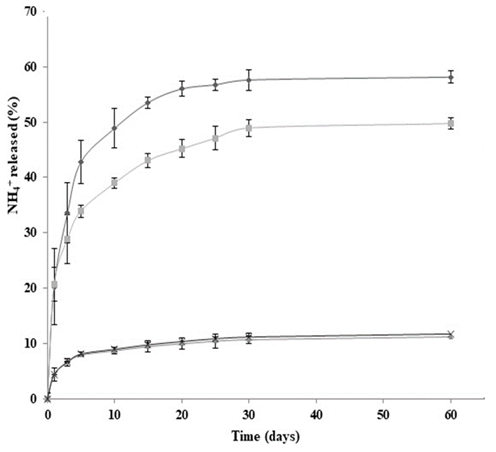
Fig. 2 shows the release profiles for NH4 + (diammonium phosphate and hydrogel) from day 0 to day 60 of the evaluation period.
Source: The authors
Figure 2 Release of NH4+ in water:  diammonium phosphate in soil,
diammonium phosphate in soil, 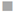 diammonium phosphate in sand;
diammonium phosphate in sand; 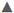 hydrogel in soil,
hydrogel in soil,  hydrogel in sand.
hydrogel in sand.
During the first five days of evaluation, diammonium phosphate released 43 ± 2 % and 34 ± 2 % of NH4 + in soil and sand matrices, respectively. The high solubility of diammonium phosphate in water (57.5 g / 100 mL at 10ºC) produced this rapid release (called Tigger effect). On day 30, NH4 + release reached levels of 58 ± 2 % and 48 ± 1 %; these data are similar to those found on day 60 -this indicated that migration of NH4 + from the fertilizer to the soil matrix had reached equilibrium. During the evaluation period, larger concentrations of NH4 + were found in solutions obtained from soil (pH 4.8), than in those obtained from sand (pH 7.2); this was to be expected as pH was an essential factor in determining the solubility of nutrients in soil, where NH4 +, NO3 -, CO3 2- and SiO3 2- displayed a larger displacement of oxidation equilibria (nitrification and denitrification), and also dissociation of NH4 + ions (eq. 1 and 2) to the left side of the equation, increasing the concentration of NH4 + ions in the soil solution and favoringleaching [11]. In addition, the soil exchange complex displays a lower pH, which increases protonation at the points of exchange; this translates into positive charging of the colloidal mycelia, avoiding adsorption of NH4 + on the surface.
The different media did not exhibit statistical differences when hydrogel was used. Release of NH4 + after days 5, 30 and 60 was 8 ± 2 %, 11 ± 1% and 12 ± 2%, respectively. The first stage of release from the hydrogel can be attributed to the erosion of residual cellulose and polyvinyl alcohol chains as mentioned before [12]; structural loss also plays a role. According to the European Standardization Committee [13], a fertilizer is called “a controlled release fertilizer” when:
Once NH4 + finds its way into the substrate, water can wash it out via pore saturation, or it can remain available to plants and microorganisms [14]. Although the release profile in water does not represent the real span in which nutrients exit soil, it allows for comparison between commercial fertilizers and the hydrogel. In this way, we can test the capacity of our hydrogel to release NH4 +.
To analyze NH4 + release data, we used the Korsmeyer-Peppas empirical model [15] (eq 3), for polyvinyl alcohol hydrogel controlled-release systems in soil [16,17].
In eq 3, 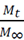 is the fraction of drug or fertilizer used by time t, K is the constant that marks the speed of release and n is the release exponent.
is the fraction of drug or fertilizer used by time t, K is the constant that marks the speed of release and n is the release exponent.
To estimate n and K values, eq. 3 was linearized
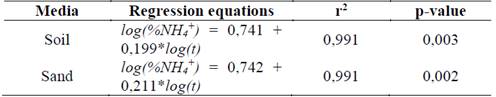
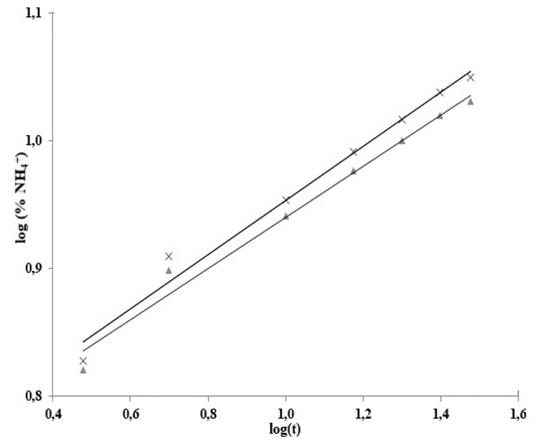
Fig. 3 shows log (% NH4 +) versus time, Table 3 shows the statistical results of NH4 + release data linearization. The data exhibits significant statistical correlation (p-value < 0.05) between log (NH4 +) and log (t), with a 95% confidence level; the correlation coefficients for the models used were larger than 0.99 -this indicates a strong correlation between variables.
Source: The authors..
Figure 3 Adjustment to the Korsmeyer-Peppas model for the release of NH4
+ in water: 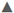 hydrogel in soil,
hydrogel in soil, 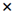 hydrogel in sand.
hydrogel in sand.
From the equations shown in Table 3, the following equation was obtained:
Using the antilog function on both sides of the equation:
Since k is constant then 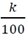 must also be a constant:
must also be a constant:
In this way, the leaching of NH4 + in water adjusts to the Korsmeyer-Peppas model [18](eq. 3), which adequately describes the leaching process. This model explains the NH4+ release mechanisms for residual-cellulose/polyvinyl-alcohol hydrogel; the derived n and K values can be seen on Table 4.
Table 4 Parameters of the Korsmeyer-Peppas model for release of NH4+ in water by leaching from RC/PVA.
Source: The authors.
For polyvinyl alcoholhydrogels with different degrees of hydrolysis, a Fickian diffusion mechanism (n = 0.45) was proposed. For hydrogels with chitosan, xanthan and starch contents the mechanism proposed is not Fickian (0.45 < n < 0.89) as hydrogels disintegrated easily.
Hydrogel shows no significantly different n values for treatments in soil and sand, but values of n < 0.45 indicate a lower rate for water infiltration into the hydrogel than the rate at which its polymeric chains can relax. This phenomenon has been attributed to the high content of calcium carbonate in the hydrogel’s structure; even so, the process remains Fickian -such behavior is called “Less Fickian” [19,20].
3.2. Evaluation of the hydrogel in greenhouse conditions
Fig. 4 shows the control (left), diammonium phosphate-treated (center) and hydrogel-treated (right) samples used in the greenhouse tests; it is evident that plants in the right-hand side column are taller and display broader leaves.

Source: The authors.
Figure 4 Zea mays parts and treatment used in greenhouse tests: a) leaf (control), b) leaf (diammonium phosphate); c). leaf (hydrogel), d) root (control), e) root (diammonium phosphate); f) root (hydrogel).
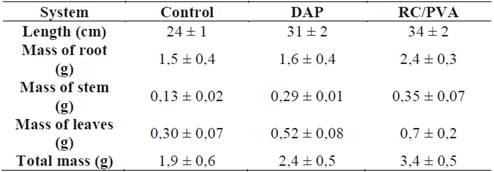
Table 5 shows the aver age heights of the hydrogel-treated plants and the diammonium phosphate-treated plants; p > 0.05 suggests there are no significant differences in the treatments. However, both treatments are significantly different from the control group. The mass of the plants grown in hydrogel-fertilized soil was larger than that of diammonium phosphate-fertilized plants; these findings show the positive effects of hydrogel-fertilized soil on the bioindicator. The results shown confirm that nitrogen fertilizers can be optimized, averaging lower production costs and reducing environmental impact produced by traditional fertilization processes [21].
3.3. Analysis of the morphological changes in the Hydrogel
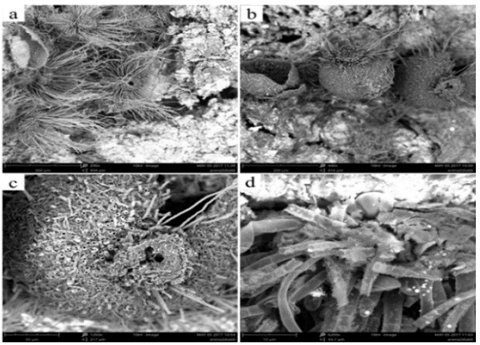
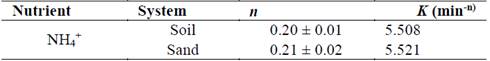
Hydrogel produced the largest release of NH4 +. When hydrogel interacts with solid fractions in soil (organic and inorganic) it releases NH4 +, but the rate at which that release takes place is dependent on the degradation of the cellulose matrix. Degradation was verified on day 60 using scanning electron microscopy (SEM). Fig. 5b shows the presence of a microorganism growing on the surface of the hydrogel; given the characteristics of the material, microorganisms can exhibit cellular activity and contribute to its degradation.
Source: The authors.
Figure 5 Scanning electron microscopy (SEM) of residual cellulose/polyvinyl alcohol hydrogel: a) before application to soil, 950x and b) 60 days after application, 290x.
Fig. 6 shows a filamentous fungus with cylindrical hyphae, spherical structures, ascomata (flask-like, some already broken due to the release of ascospores to the media) and conical-shaped porous hyphae (characteristic of the ascomycota) [22].
3.4. Microbiological characterization of the soil isolates
After 60 days, we isolated (in 4 % Glucose Sabouraud agar) from hydrogel-fertilized soil, a fungus with the same characteristics as those found on the hydrogel’s surface. None of the tests performed revealed the presence of enterobacteria or E. coli (both regulated by Colombian legislation and the National Health Institute).
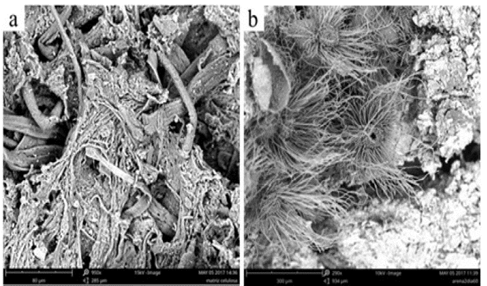
On the sixth day of isolation (Fig. 7a), an unlimited number of white cottony colonies was discovered throughout the Petri dish, all of which exhibited radial growth. On the backside of the plate, a diffused brown shade appeared (Fig. 7b). After ten days of isolation (Fig. 7c) the cottony colonies, once white, turned purplish, and then covered the entire agar, adhering to the walls. Meanwhile, the brown shade on the back of the plate turned pink (Fig. 7d).
Source: The Authors.
Figure 7 Development of the isolated fungus on Sabouraud agar; a) Cottony mycelia on day 6, b) Backside of the plate on day 6, c) Cottony mycelia on day 10 and d) Backside of the plate on day 10.
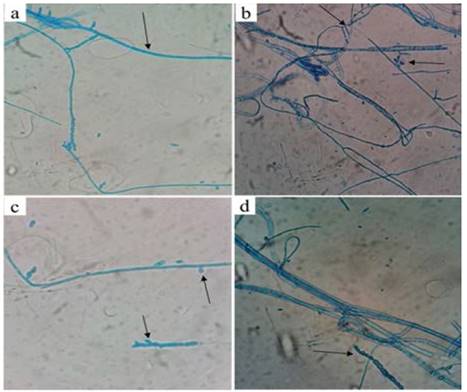
Fig. 8 shows images (at 40x) of the culture plates in lactophenol blue. On the images we observe the fungus’ mycelium, composed of thin septate hyaline hyphae (Fig. 8a), lateral phialides (Fig. 8b), oval and ellipsoidal microconidia and ascospores (Figs. 8c and 8d) [22]. The macroscopic and microscopic taxonomic characters identified in the fungal isolate match those of Fusarium spp.
4. Conclusions
NH4 + release data adjusts well to the Korsmeyer-Peppas empirical model, suggesting a Fickian diffusion mechanism.
A controlled-release system was manufactured to improve nutrition in plants (especially Zea mays). Increased height and biomass in the plants indicate a substantial contribution of NH4 +; this makes the hydrogel a compelling alternative to traditional chemical fertilization processes. It is, however necessary, to adjust first the conditions that facilitate the application of this technology to different types of plants and soils.