1. Introduction
Polyhydroxyalkanoates (PHA) are fully biodegradable biopolymers of microbial origin with physiochemical characteristics similar to those of conventional synthetic plastics, from rigid and brittle plastics to rubber-like plastics [1-5]. There are different microbial strains employed in the production of PHAs; Rasltonia eutropa, for example, can metabolize different carbon sources and accumulate the biopolymer [6,7].
Despite the environmental advantages that PHAs represent and their wide field of application in comparison with petrochemical plastic, their use and industrial profitability is limited by high production costs which, in 2010, reached estimated values of 6.25 USD/kg, surpassing prices of petroleum-based polymers, which had reached an estimated cost of 1.45 USD/kg [8], preventing them from being economically competitive.
Much of PHAs high costs respond to the culture medium employed, which accounts for up to 50% of the total production cost [9]. In view of this, the study and evaluation of low-cost non-conventional substrates to elaborate such a culture medium is increasing.
Molasses emerge as a good alternative because of its nutritional composition, rich in sugar, protein, amino acids and microelements such as Mg, P and K. Given the large extent of sugarcane crops in Colombia, it is possible to obtain residual molasses, from the sugar refinement process, at a low cost [10]. Vinasse, another residue obtained from ethanol distillation processes, is rich in salts [11]. Therefore, it could be used as a complement to the elaboration of culture media for fermentative processes.
The use of vinasse in PHA production could reduce its negative impact on the environment since it would reduce the amount of water and salts necessary for the elaboration of the culture medium and its effects if not properly disposed, caused by its high levels of BOD and COD between 35,000-50,000 and 100,000-150,000 mg O2/L respectively [11,12], which exceed the permissible discharge limits for the production of agroindustrial activities (sugar and derivatives from sugar cane) and for non-domestic wastewater discharge - nDWD to surface water bodies, BOD 500 mg/L O2, COD 900 mg/L O2 [13], according to Resolution 0631 of 2015 (Ministry of Environment - Colombia). All things considered, this study evaluated different molasses/vinasse ratios to obtain a non-conventional culture medium for PHA production by Rastonia eutropha ATCC 17699, given its ability to metabolize different carbon sources.
2. Materials and methods
2.1. Microbial strain and culture condition
Ralstonia eutropha ATCC 17699 (Cupriavidus necator) was purchased from ATCC (Manassas, VA, USA) through CES University. The bacterium was cryopreserved at -20 °C in tryptic soy and broth medium (TSB) in 30% glycerol, inside a 1.5 mL vial, and subcultured in TSB under standard monthly conditions.
The inoculum used for culture in the reactor was carried out in 1L Erlenmeyer flasks containing 300 mL of TSB medium, at 30°C and 150 rpm for 12 hours, using a vial with the bacterial strain cryopreserved.
The culture was carried out in a 5L bioreactor (New Brunswick) with automatic control of agitation at 200 rpm, a temperature of 30oC, air flow of 5 L/min and pH 7.0, controlled with 1N HCL and NaOH solutions.
2.2. Treatment and characterization of raw materials used as a substrate
The vinasse for experimentation was donated by a sugar-alcohol company in Colombia. It was centrifuged at 8,000 rpm for 7 min to remove particulate matter. The supernatant was then characterized to determine total sugar, Ca, Mg and K content. On the other hand, commercial molasses employed (high viscosity sugar syrup) contained 60-63 % (w/w) sucrose and 3-5 % (w/w) reducing sugars. Due to the high sugar content, this was diluted to obtain the ratio and concentration of sugar required for experimentation.
Mineral salt medium (MSM) employed in the culture as a reference medium contained (per liter): Glucose 20 g; Na2HPO4.7H2O 6,7 g; KH2PO4 1,5 g; (NH4)2SO4 1,0 g; MgSO4.7H2O 0,2 g; iron and ammonium citrate 60 mg; CaCl2.2H2O 10 mg; element trace solution 1 ml. Element trace solution (per liter): H3BO3 0,3 g; CoCl2.6H2O 0,2 g; ZnSO4.7H2O 0,1 g; MnCL2.4H2O 30 mg; NaMoO4.2H2O 30 mg; NiCl2.6H2O 20 mg; CuSO4.5H2O 10 mg [14].
2.3. Biomass determination
Biomass concentration was determined by gravimetry, using dry weight determination with 5mL samples of culture broth. The samples were passed through membrane filters (Millipore, 0.45 µm filters) in a filtration equipment with a vacuum pump, to be then dried at 70°C for 24 hours and weighed in an analytical balance to constant weight.
2.4. Sugars determination
A high-performance liquid chromatography (HPLC) Agilent Technologies 1200, model 61362A, with a column of separation ionic interchange Animex HPX-87H, 300x7.8mm was employed in sugars determination. The samples were centrifuged at 7,000 rpm for 5 min and aliquots of the supernatant were taken, which were diluted in mobile phase according to the concentration range of the standard curve employed. Each diluted sample was run through 0.2 µm regenerated cellulose filters and injected under the following conditions: 20 µL sample, 0.6 mL/min flow, 35oC and 12 min run time. All samples were analyzed in triplicate.
2.5. PHA extraction, and characterization by FTIR
The extraction of the PHA produced was carried out following Yu & Chen methodology, where the sample was subjected to a sterilization process at 120oC for 30 min, to be then is recovered by centrifugation at 5,000 g for 12 min and washed with distilled water; then, 200 mL of 0.1 M H2SO4 are suspended in solution and subjected to digestion at 100 oC for 2 hours, adjusted to pH 10 with 5N NaOH and centrifuged at 4,000 rpm for 20 min to recover the biopolymer. This is resuspended in a 6% NaCl solution to be bleached and is again recovered by centrifugation and washed with distilled water. Determination of the PHA production was performed using dry basis quantification [15].
For the characterization of the PHA produced, Fourier Transform Infrared Spectroscopy (FTIR) technique was used, with a Perkin-Elmer Spectrum BX infrared spectrophotometer and ART module from 4,000 to 400 cm-1 and a Sigma-Aldrich branched PHB (Polyhidroxibutyrate) standard with 99% purity.
2.6. Experimental design and statistical analysis
Three one-way experimental designs were performed. The first factor was evaluated with different relations of molasses/vinasse (M/V: 100/0, 75/25, 50/50, 25/75); the second factor was the nitrogen source (with and without nitrogen source); the third factor was the salts supplement (with and without salts supplement). The nitrogen and salts from the MSM medium were used as a reference for the second and third factor. All experiments were carried out in triplicate, using biomass, sugars, and biopolymer as response variables.
A one-way variance analysis (ANOVA) and a Tukey HSD multiple comparisons test were performed to evaluate the data. The results that presented values of p-value < 0.05 were interpreted as statistically significant variables using the software StatGraphics Centurion 17.1 Demo.
3. Results and discussion
3.1. Characterization of vinasse
Vinasse was characterized according to available sugar content, which was quantified by HPLC; this, together with other components that can be seen in Table 1, were analyzed at the Laboratory of Physicochemical Analysis, Institute of Chemistry (Laboratorio de Análisis Fisicoquímico del Instituto de Química - LAFQ) , University of Antioquia.
According to Table1, the sugar content in the vinasses reached 11.64 g/L, so it was required to complement the medium with an additional carbon source such as molasses, according to the carbon requirements of the MSM. The pH also needed to be adjusted but Ca, Mg and K contributions exceeded those required by the reference medium [14]. However, Zuñiga et al. (2013) report pH values in vinasse of 4.6-4.7 and 7.4 - 8.4 g/L in total solids [11], in agreement with the values in this work.
3.2. Evaluation of different molasses/vinasse ratios
Table 2 shows the concentration of sugars consumed by R. eutropha under different molasses/vinasse ratios. The growth of bacteria in all cultures was evidenced by microscopy. It was also observed that the highest yield of total sugars was reached in the proportion 25/75 molasses/vinasse, presenting a statistically significant difference between the other proportions (p-value <0.05). Similarly, Dos Santos et al. reported a yield of 0.79 or 79 % of sugars consumed in an R. eutropha culture, using vinasse obtained from cane ethanol production [16].
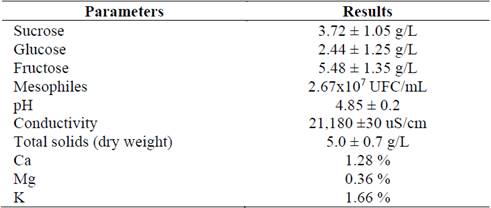
Fig. 1 shows a profile of time-quantified sugars for the cultivation of R. eutropha at an M/V ratio of 25/75 with supplementation of nitrogen and salt sources (1.0 g/L of (NH4)2SO4) and according to the MSM culture medium. It also shows the consumption of total sugars (sucrose, glucose, and fructose), the gradual consumption of sucrose, and how at 18 hours post-culture the total glucose had been consumed.
Table 2 Consumption of sugars performance under different proportions of molasses/vinasse in cultures of R. eutropha ATCC 17699 in the 5L bioreactor.

Source: The authors.
Source: The authors.
Figure 1 Consumption of sugars by R. eutropha ATCC 17699 of an M/V ratio: 25/75, with nitrogen and salt supplementation, in the 5L bioreactor.
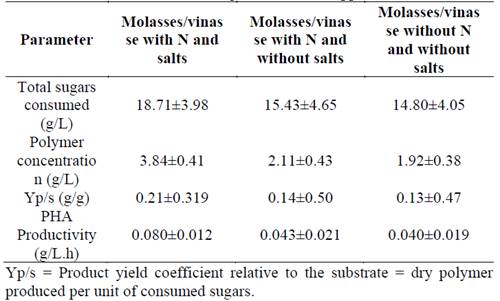
The results in Table 3 correspond to the evaluation of the culture supplemented with nitrogen and salt sources, with M/V of 25/75. Although there were no statistically significant differences between treatments, PHA productivity, Yp/s and consumption of sugars were higher in the mixture supplemented with nitrogen and salt sources.
Table 3 Experimental results of R. eutropha ATCC 17699 in molasses/vinasse: 25/75 culture with or without nitrogen and/or salt supplementation.
Source: The authors.
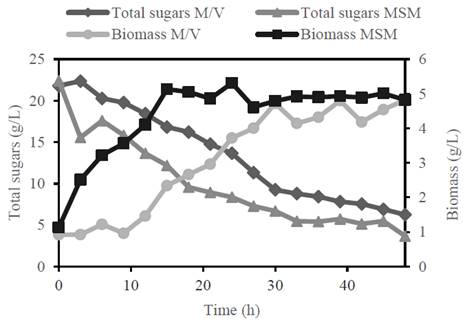
Finally, in Fig. 2, a comparison was made between the MSM reference culture and the culture using an M/V ratio of 25/75 supplemented with nitrogen and salt sources. In both cultures, the consumption of total sugars with values of 18.60 and 15.56 g / L is appreciated, which represents an efficiency in the consumption of sugars of 83.1 and 71.3%, respectively.
Source: The authors.
Figure 2 Growth of R. eutropha ATCC 17699 in molasses/vinasse: 25/75 with addition of nitrogen and salts and in MSM reference medium, grown in a 5L bioreactor.
In the culture with molasses/vinasse, a lag phase of 9 hours could also be observed, unlike the culture with the MSM medium. This difference in the lag phase may be related to the low concentration of glucose in the medium forcing R. eutropha to hydrolyze the available sucrose.
Table 4 shows the results of the parameters determined for each crop; no statistically significant differences were found between the parameters evaluated except the total of sugars consumed. In this way, the M/V medium can be considered suitable for the cultivation of R. eutropha, with the capacity to accumulate up to 97.8 % of PHA with respect to the produced biomass and a yield of 97.8 % in total sugars consumed. However, Nonato et al. cultivated R. eutropha with a medium enriched with cane molasses with a productivity of 1.44 g PHA/L.h, but with a biopolymer content of 65-70% [17].
Table 4 Comparison of the parameter for R. eutropa ATCC 17699 in MSM and culture with M/V: 25/75 supplemented with nitrogen source and salts.
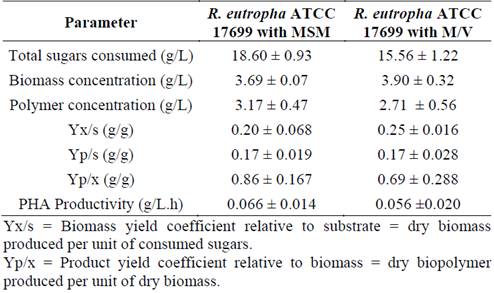
Source: The authors.
Other researchers, such as El-Sayed et al., also carried out cultures with mixtures of glucose and sucrose obtaining 0.8 g/L PHB, but with a change in the culture, strategy reached 7.31 g/L PHB [18]. On the other hand, Pramanik A et al. also used vinasse as a culture medium for the production of PHAs but using the strain Haloarcula marismortui, with which a content of 23-30% was obtained in the dry biomass with a productivity between 0.015 and 0.020 g / L.h of polymer [19].
3.3. Biopolymer characterization by FTIR
By comparing the spectra of the polymers obtained in Fig. 3 with the R. eutropha strain versus the polyhydroxy butyrate standard (PHB), an equal pattern is observed in the formed bands. The peaks corresponding to the region of the spectrum of 1,740 cm-1 and 2,970 cm-1 are characteristic of C = O and CH3 functional groups of the monomeric PHB [20].
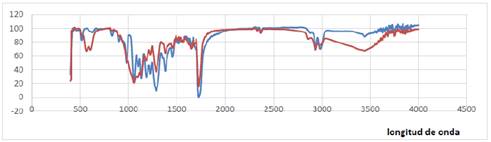
Source: The authors.
Figure 3 Spectra of the polymer produced with R. eutropha ATCC 17699 in the medium MV and the reference polymer PHB brand Sigma-Aldrich, using the Fourier transform infrared spectroscopy (FTIR).
The spectra obtained from R. eutropha showed a correlation of 99.25% with the pattern of bands in the PHB commercial polymer employed, indicating thus that the polymer obtained from R. eutropha is of the PHB type [21] [22]. According to reports, this polymer is highly crystalline (> 50%). The melting and glass transition temperature is about 180 ° C and 4 ° C, respectively. It has some mechanical properties comparable to degradable synthetic polyesters, such as PLA. During the storage time at room temperature, it undergoes a secondary crystallization of the amorphous phase, as a result, the tensile and elongation modulus increase while the polymer becomes more brittle and harder [23] [24].
This biopolymer is an attractive material for tissue engineering due to its inherent biocompatibility. It has been shown to enhance the proliferation of different cell types and since PHB is susceptible to various processing methods, it can be mixed with other types of biocompatible molecules such as the hydroxyapatite mineral to create films, fibrous mats or non-crystalline chains, adaptable to a specific use [25] [26].
4. Conclusions
Both molasses and vinasse can be used as a non-conventional culture medium in the production of PHA, thanks to their contributions to carbon and micronutrient sources.
Vinasses alone do not provide a sufficient carbon source to achieve high amounts of biomass and PHA production. Therefore it is essential to supplement the culture with an additional carbon source.
The most suitable proportion of the molasses/vinasse mixture was 25/75, achieving a substrate consumption efficiency of 83.6%. On the other hand, R. eutropha ATCC 17699, has the ability to consume sucrose as a substrate.
The FTIR analysis between the PHB Polyhydroxybutyrate sample and the polymer produced by R. eutropha showed a correlation between its bands of 99.25%, which allows us to state that the biopolymer produced was of the polyhydroxybutyrate type.