1. Introduction
The increasing trends toward device miniaturization and the demand for novel materials to face challenges in the treatment of specific illnesses have sharpened scientific interest in the potential of nanotechnology, in which nanostructures exhibit size-dependent properties [1,2]. In particular, this interest is directed toward nanomultifunctional materials that combine certain properties (e.g. magnetic, electric, and optics, among others) to yield a single device component with improved functionality [3]. One of the structures that can be used to obtain multifunctional materials is the core/shell, which consists of a core material surrounded by another one with similar or different properties. In many applications, nanoparticles with a core/shell structure are preferred because control of their chemical composition and of the relative size of the core and the surface shell offer possibilities for tuning their properties [4,5]. Core/shell nanoparticles have been the subject of extensive scientific research because of their potential in the biomedical and biological fields, such as hyperthermia in tumor therapy [6], drug delivery, magnetic resonance image [7], and regulation of cellular process through control of ion channels [8]. These applications stem from the nature of the core/shell nanoparticle, comprising magnetic nanoparticles (MNPs) or magnetoelectric nanoparticles (MENs).
MNPs are the basis of magnetic fluid hyperthermia, where the nanoparticles undergo the action of an externally applied alternating field in order to generate a certain amount of heat, proportional to the frequency of that field [9]. By contrast, in conventional hyperthermia the external heat source uses ultrasound, radiofrequency, microwave, infrared radiation, or tubes with hot water to transfer heat [10]. The principal advantage of MNPs in hyperthermia treatment is the local rise of temperature while preserving the healthy tissue, because the frequencies of the oscillating magnetic fields that are generally utilized pass harmlessly through the body and generate heat only in tissues containing MNPs [11]. Among the conditions that MNPs must meet, high biocompatibility, low toxicity, small size, and a high degree of magnetization controlled by an external magnetic field should be mentioned. Also, MNPs can be used for trigger-controlled delivery of drugs in the presence of oscillating magnetic fields [12,13]. Indeed, magnetic functionality has been explored in order to utilize MNPs as a diagnostic tool (primarily in magnetic resonance imaging) and as a targetable drug carrier for therapy, a so-called theranostic [14].
Another relevant application of core/shell structures is combining magnetic and electric materials in nanoparticle morphology, thus obtaining MENs. These structures have promising applications in biomedicine, such as in the stimulation of functions of living cells and as carriers for drug release [15,16]. It is worth mentioning that multiferroic materials can possess at least two ferroic properties, such as ferroelectricity, ferromagnetism and/or ferroelasticity, although more attention has been paid to applications in the study of ferroelectric and ferromagnetic systems, which are linked to the piezoelectric and magnetostrictive phases, respectively [17]. The challenge of implementing the application of multiferroics is tied to the main multiferroic phenomenon called the magnetoelectric (ME) effect, which arises from the stress transfer in the coupling of piezoelectric and magnetostrictive phases through the interface [18]. Thus it allows switching the polarization by the application of a magnetic field (direct ME effect) or magnetization when an external electric field is applied (converse ME effect) [17]. Here the direct ME effect is the main mechanism in biomedical applications of MENs, in which the ME voltage coefficient is estimated as a reference to the electric field generated to control the ion channel [19]. On the other hand, in data storage, multiferroic vertical core/shell structures that consist of ferrimagnetic nanopillars embedded in a ferroelectric matrix have been proposed for electrically assisted magnetic recording [20]. Thus all the above shows the important role of core/shell structures in nanoscale applications.
Therefore, we present a review of the current state of research in core/shell nanoparticles with magnetic and multiferroic properties, and their potential applications, mainly focused on the field of biomedicine.
2. Core/shell nanoparticles with magnetic properties
Many combinations of core/shell nanoparticles composed of polymers, biomolecules, silicas, metals, and metal oxides have been explored, mainly designed for biomedical applications, based on their surface chemistry, which increases their affinity for binding with drugs, receptors, ligands, etc. [21,22]. For instance, the clinical use of magnetic hyperthermia as a therapeutic treatment requires MNPs with a high heating potential, which is quantified by the specific absorption rate (SAR), because it involves a reduction of the ferrofluid dose in vivo and the treatment of small tumors. A particular strategy for increasing the SAR or heating efficiency is using core/shell structures, in which a metal core such as iron, gold, or silver is commonly used, among others, but because of the toxicity and instability of pure nanoparticles, iron oxides are mainly used as shell [23], as well as compounds of ferrites. The core must exhibit a high saturation magnetization in order to obtain a high heating effect, surrounded by a biocompatible magnetic layer in order to protect the core from oxidation or leaching, and further to functionalize the surface for imaging, targeting, or sensing applications [24].
The heating efficiency value depends on parameters such as the magnetization, the size and size distribution of particles, the strength of the magnetic field, and the frequency of the alternating magnetic field [25]. Also, these factors determine the main mechanisms ascribed to magnetic heating in nanoparticles: hysteresis loss and susceptibility loss, in which the SAR is calculated from the area of hysteresis loop multiplied by the frequency and from the Neel-Brown relaxation model, respectively. A large number of studies, including both experimental and theoretical ones, have been carried out in order to analyze the effect of of the parameters of the nanoparticles on the SAR. So in the following sections, a review of the nanoparticle properties for use in hyperthermia will be presented, as well the recent experimental/theoretical results for core/shell structures.
2.1. Magnetic regime
The magnetic properties of materials fundamentally change when the size of the particles is reduced [26]. These changes can be observed in the domain configuration through the area of hysteresis loops, because as the size of a ferromagnetic or ferrimagnetic material decreases, it can pass from a multidomain state (the configuration for the bulk), to a single domain, and finally, below a critical size, it becomes superparamagnetic (see Fig. 1). For biomedical applications, a high saturation magnetization (Ms) is ideal, because it involves a large thermal energy dissipation [27]. In this way, the particles must have reduced size and be in the single domain or the superparamagnetic state for biomedical applications. In single domain regime, typically between 20 and 100 nm, the nanoparticles presents the highest heating efficiency, depending on the material and particle shape [28].
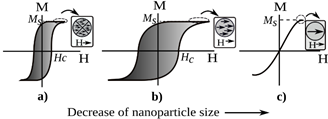
Source: The authors.
Figure 1 Effect of size of particles on magnetic behavior. a) Multidomain, b) single domain, c) superparamagnetic.
While, despite the low SAR values in superparamagnetic nanoparticles, once in vivo these particles are magnetic only in the presence of an external field, giving them a unique advantage for working in biological environments [29]. Also, this state prevents the agglomeration of nanoparticles therefore the potential embolization of capillary vessels [30].
2.2. Size effect
Many studies have been carried out to establish the effect of size on the SAR value and the optimum size to maximize it. However, the appropriate size is still a matter of debate, due to the lack of a relationship between in vivo results and the estimated ones for a wide range of tumors [31], and their variation with the amplitude of applied magnetic field [32]. For biological applications, nanoparticles under 100 nm are considered appropriate for tissue penetration, but for hyperthermia, the minimum size is approximately 30 to 40 nm [31]. Nevertheless, there is not a consensus in this range, as other authors suggest that the preferred size is typically around 10-50 nm [27].
A significant size effect was found by Nemati and coworkers for core/shell Fe@Fe3O4 nanoparticles. They pointed out that sizes below 14 nm can change the core/shell to a hollow structure because of a higher diffusion of cations than anions over time in a process called the Kirkendall effect, which is size dependent. It causes a considerable deterioration of the magnetic properties and the heating efficiency [33]. Furthermore, according to the size, the alternating magnetic field leads to an increase in heating due to Neel or Brownian relaxation processes or hysteresis losses, and so it becomes a critical tuning parameter [34].
Likewise, the decrease in MNP size affects a prominent factor called magnetic anisotropy energy that maintains the magnetic moment in a particular orientation, causing a decrease until it reaches a state where the magnetic moment can flip randomly, due the fact that the anisotropy becomes equal to the thermal energy. This is the superparamagnetic regime [34]. The improvement of anisotropy energy for superparamagnetic nanoparticles can increase the energy loss as because of the blockage of the magnetization [31]. Although other reports have indicated an enhancement of heat dissipation in large ferromagnetic nanoparticles with low anisotropy [27,35].
Moreover, it was showed by the experimental comparison of SAR values for different synthesis approaches that the relationship between the SAR and the size is not linear, as was presented by G. Salas et al. [30], suggesting that the SAR reaches a maximum and then for large sizes it decreases, for a given amplitude and frequency of the applied magnetic field.
2.3. Size distribution effect
The sizes of the MNPs in the ferrofluid also establish the SAR values. Different heat efficiencies can occur for MNPs with non-similar sizes, because these can present distinct saturation magnetisation and anisotropies [30]. In order to obtain a more homogeneous heating effect for hyperthermia, monodispersed (or a narrow size distribution) nanoparticles are desirable [30,36]. In this way, experimental methods, have been achieved to reach nanoparticles with similar average size. For this propose wet-chemical methods [32,37] and techniques such as thermal decomposition [38,33] and chemical precipitation [39] have been employed. Nevertheless, any experimental system has some unavoidable polydispersity, resulting in a log-normal size distribution of nanoparticles with a standard deviation 𝜎, where 𝜎≈0.1 depicts a monodispersed system [40]. In this distribution for 𝜎 between 0.2 and 0.25 the dissipated energy can drop between 30% and 50% [41].
However, fewer size distribution effect studies are reported in core/shell systems compared to single-phase nanoparticles. For instance, in core/shell systems conformed by a magnetic core (commonly iron oxide based nanoparticles) and a shell of silica, the core size were varied between 5 nm and 110 nm. As a result, a higher SAR (137 W/g) was obtained for nanoparticles of average size of 24 nm, in contrast for 110 nm (1 W/g) where the nanoparticles drop in multidomain state [42]. Also, in Fe@Fe3O4 a narrow size distribution (10-15 nm) in single-domain range led to high SAR response [43]. Likewise, CoFe2O4@MnFe2O4 and MnFe2O4@CoFe2O4 core/shell nanoparticles were synthesized by means of thermal decomposition, to obtain high monodispersity (𝜎<0.1) and small size distributions (less than 30 nm), yielding a high thermal efficiency, even with the concentration increase, comparing to single-phase (CoFe2O4 and MnFe2O4) nanoparticles [44].
2.4. Shape effect
Several studies with a modified shape have been conducted in order to observe the effect on the SAR value. For instance, M. Vasilakaki et al. studied the effect of shape and size on SAR values for magnetic core/shell nanoparticles of Fe@Fe3O4, by using the susceptibility losses for calculating the SAR from magnetic properties obtained by Monte Carlo simulation. It is important to emphasize that they modified the model in order to calculate the SAR by adding the volume and saturation magnetization of each layer (core, shell, core/shell interface), although for all shapes (cubic, spherical, octahedral and truncated cuboctahedral), they observed high SAR values compared with Fe3O4 experimental nanoparticles. The highest value was exhibited by the truncated cuboctahedral that is attributed to a lower volume, in contrast to cubic geometry [45]. By contrast, an experimental study of FeO@Fe3O4 cubic and spherical nanoparticles showed that a higher saturation magnetization (in the spherical geometry) is not the only factor for determining the SAR, but that the effective anisotropy (shape and exchange) also produce a higher SAR for the cubes as compared to the spheres [46]. So, based on the studies in the literature, a clear conclusion in favor of a particular shape cannot be reached; however, its effect on the magnetic and thermal properties is notable.
2.5. Synthesis of magnetic core/shell nanoparticles
A high rate of progress has been seen in the experimental growth of core/shell nanoparticles over the last decade, allowing control of their size and shape. Recently, Khurshid et al. [46] prepared spherical and cubic FeO@Fe3O4 nanoparticles via the thermal decomposition method. The FeO@Fe3O4 composition and the core/shell geometry was confirmed transmission electronic microscopy (TEM), as is shown in Fig. 2. Measures of hysteresis loops indicated higher saturation magnetization for spheres than for cubes; however a better effective anisotropy for cubes yielded a higher SAR compared to spheres, indicating that anisotropy is an important parameter in magnetic hyperthermia. Previously, these researches presented an interesting route to synthesize core/shell FeO@Fe3O4 with a controlled size and shape, supported in the variation of conditions in a chemical reaction. They found that nanoparticle size can be changed by controlling the concentration of surfactants, while the shape is modified by their molar rations, during the reaction

Source: Adapted from [46]
Figure 2 TEM images of the FeO@Fe3O4 nanoparticles for distinct shapes: a) spheres, b) cubes.
Likewise, for Fe@Fe-oxide obtained by co-deposition with water vapor from the gas-phase, the control of shell nanoparticle was possible by changing the temperature of shell vapor [47].
The exchange coupling between a magnetically hard core (CoFe2O4) and a magnetically soft shell (Ni0.5Zn0.5Fe2O4) has also been explored as a gauge of conversion efficiency from electromagnetic to heat energy, because it allows an optimal tuning of magnetocrystalline anisotropy K. For this purpose, the core/shell was synthesized by a reflux method. The K value was calculated with the saturation magnetization and the coercivity field of hysteresis data, from the Stoner-Wohlfarth theory. They observed that CoFe2O4,@Ni0.5Zn0.5Fe2O4 nanoparticles are magnetically exchange coupled because the coercivity field fell between the values for CoFe2O4, and Ni0.5Zn0.5Fe2O4 nanoparticles, which causes the tuning in K value that increases the SAR [48]. In these experiments some of the values employed of the frequency (f) and the amplitude (H) of magnetic field satisfied the safety human range (Brezovich criterion) in which f must be lower than 1.2 MHz and H below 15 kAm-1, and the product f*H not exceed 5x109 Am-1s-1 [9].
Other important factors in the heat efficiency are the biocompatibility and stability that are given by the surface of core/shell nanoparticle. According to Zhang et al., the modification of surface chemistry provides a way of optimizing the particle’s properties. In their research, iron/iron oxide nanoparticles were prepared by microemulsions, and then the surface of nanoparticle was modified with a hydrophic layer (it prevents oxidation of metallic core, and improves lifetime in aqueous environments, as is depicted in Fig.3) of hexamethyldisilazane (HMDS) and a phospholipid coating PC (it provides a biocompatibility coating), enhancing the quality of nanoparticle and the hysteresis losses, being suitable for hyperthermia [49].
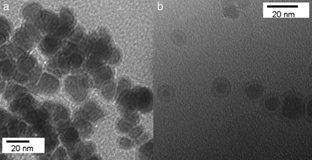
Source: [49]
Figure 3 TEM images of Fe/Fe3O4/HMDS/PC nanoparticles: (a) without exposure to water; (b) exposed to water for 1.5 h.
3. Core/shell nanostructures with multiferroic properties
3.1. Voltage-gated ion channels
Stimulation of the vital functions of living cells can be activated by the interaction of ion channels with electromagnetic fields. Ion channels are membrane proteins that form pores for controlled exchange of ions across cellular membranes [50]. Their two main characteristics are their selectivity, i.e. the type of ions they flux (e.g. K+, Na+, or Ca++) and their gating, i.e. the process of opening and closing in response to the so-called gating variable [8]. It has been identified that defects in ion channels are the cause of human and animal diseases such as cystric fibrosis, diabetes, cardiac arrhythmias, neurological disorders, hypertension, etc. [51]. One way to trigger changes in ion channel gating was proposed by Kargol et al., which is based on the use of multiferroic nanoparticles to induce modifications in the electric fields by the application of an external magnetic field [8]. For this, the nanoparticles can be targeted to a specific location by the action of the antigens on the surface of the nanoparticle and through the application of a magnetic field. So, when the magnetic field is applied to the nanoparticles, which are close to the cells (extracellular or intracellular), it causes a mechanical deformation in the magnetic component of the nanoparticle (because it has the magnetostrictive property). This stress is transferred to the electric constituent, which causes changes in the electric field, since the electric phase is piezoelectric, as illustrates Fig. 4. This mechanism exhibits the direct ME effect, where the ME voltage coefficient is the main parameter that indicates the coupling between the magnetic and electric phases. The induced electric field for the stimulation of the ion channel will be on the order of several mV [8].
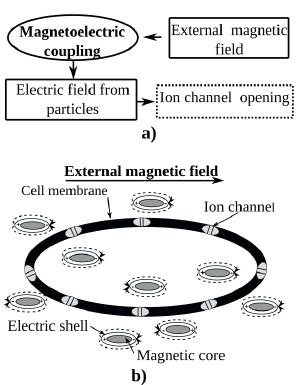
Source: Adapted from [9]
Figure 4 Mechanism of ion channel stimulation according to Kargol. a) The magnetic fields cause local electric fields due to the magnetoelectric coupling in multiferroic nanoparticles. b) Core/shell multiferroic nanoparticles generates the activation of ion channels.
3.2. Data storage
Although the core/shell nanoparticles have a strong potential for biomedical applications, these structures are also promising for electronic devices such as magnetoelectric memories. For this aim, a significant increase in ME coupling has been observed in composites, which are combinations of ferromagnetic and ferroelectric materials, compared with single-phase materials [52,53]. Over the last few decades, scientific purpose has been focused on enhancement and control of the ME coupling in composites by exploring experimental behavior. According with the current findings, some of the requirements for reaching a strong ME coupling in composites good mechanical contact in order to transfer the strain between phases [54], a large piezoelectric coefficient for ferroelectric materials (i.e. Pb(Zr,Ti)O3 (PZT), PbTiO3 and BaTiO3), and for magnetic materials a high magnetostrictive parameter (e.g. Tb1−xDyx Fe2, CoFe2O4, NiFe2O4, and other ferrites), large resistivity (in order to hold the applied electric field), and good chemical stability of the phases [55]. In addition, the ME response depends on the intrinsic properties of each phase and on the mechanical coupling determined by the type of connectivity [56]. In this manner, several connective structures have been explored for improving the ME coupling, for instance particulate composites [57], laminated composites [58], multilayers [59], and ferrite nanopillars embedded in a ferroelectric matrix [60]. In laminated composites, although higher than in particulate composites, has been observed, due to the effects of a mismatch between crystal lattices, residual strains, grain boundaries, dislocations, voids, and the clamping effect of the substrate [55]. The reduction of these variables can be attained in the vertical disposition as nanopillars, which can be identified as cylindrical core/shell nanoparticles, which are more favorable for magnetoelectric coupling, since they minimize the clamping effect due to strong in-plane elastic coupling to the substrate [61]. The experimental evidence was shown by F. Zavaliche et al. [20,62] by studying the magnetization reversal in ferroelectric BiFeO3-ferrimagnetic CoFe2O4 columnar nanostructures induced by an electric field at room temperature. In one of these, they investigated thick epitaxial films with composition (BiFeO3)0.65-(CoFe2O4)0.35 grown by pulser laser deposition on SrRuO3 bottom electrode upon (001) SrTiO3 substrates (Fig. 5).
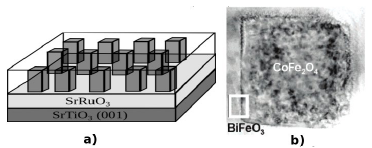
Source: Adapted from [62]
Figure 5 Vertical core/shell structures of BiFeO3-CoFe2O4. a) A sketch of the columnar nanostructures. b) TEM image of a CoFe2O4 pillar surrounded by BiFeO3.
The changes in the magnetic state before and after applying an electric field show a significant magnetoelectric coupling between the piezoelectric and the magnetic constituent of the nanostructure. Their results show the remarkable spatial control of electric field-induced magnetization switching at nanoscale for the management of data storage.
4. Conclusions
In this paper a review of some physical properties and the application scope of core/shell nanoparticles were presented. Clearly, core/shell nanostructures appear to be a promising tool for therapeutic applications, imaging, ion channel control, and information storage. An important control over parameters like size, size distribution, morphology and magnetic anisotropy, is essential for these future developments. Core/shell structures have been attained thanks to more reliable fabrication techniques, as well as theoretical contributions to understanding of the magnetic and magnetoelectric phenomena. The effect of the mentioned parameters on the SAR values for hyperthermia applications need to be investigated thoroughly, because the simultaneous interaction of different variables can hide the suitable response. In addition to the recent approaches such as the ion channel stimulation and the data storage through vertical core/shell structures, both magnetic and magnetoelectric properties, demand more experimental and theoretical investigations to fully understand variables the effect in their behavior and thus encourage the development of their applications.














