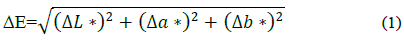1. Introduction
Fruit and vegetable juices are characterized by their high contents of dietary fiber, phytochemical compounds, vitamins, and minerals. However, because of the continuous development of functional foods marketing, there is a trend to supplement these beverages with probiotic microorganisms. The low pH of fruit makes the incorporation of probiotic microorganisms into juices challenging, as only some bacteria can only survive such conditions. Thus, bacterial strains must be chosen that are resistant to acid, and also to the cold conditions that are used during the production of probiotic fruit and vegetable beverages. The range of pH levels in food matrices can challenge the survival of probiotic microorganisms due to high levels of organic acids [1,2]. Growth characterization of probiotic strains must therefore be carried out under these conditions in order to select one which will remain viable for the entire product shelf life to provide benefits to the consumer [3]. In general, Lactobacillus species are considered resistant to acid, especially L. casei and L. acidophilus which have been seen to survive in fruit juices at pH levels between 3.7 and 4.3 [4,5].
Different studies have demonstrated that the nutrient content (carbohydrates, fiber, and proteins), antimicrobial compounds, oxygenation, and water activity have important effects on probiotic viability [6]. Studies related with the viability of L. plantarum (NCIMB 88266) in pineapple, lemon, orange, and cranberry juices, found that high fiber and protein contents had protective effects on bacterial cells during storage at 4 °C [7]. Apple and carrot are the most commonly used juices due to their positive effect on probiotic stability [8]. However, there are other fruits, for example mango (Mangifera Indica) whose composition would be optimal for probiotic survival. It has high levels of sugar (8-12 g/100 g) and is rich in minerals; carotenoids; dietary and insoluble fiber (4.2-4.6 g/100 g and 5.2-15.0 g/100 g respectively); vitamins A (1400-2500 µg/100 g), B and C (10µg/100 g, and 10 µg/100 respectively). [9,10].
In addition to matrix formulation, prebiotic inclusion is another strategy used to improve probiotic survival in functional foods. Non-digestible oligosaccharides (NDOs) have beneficial effects for host health, and have been used in foods containing probiotics because of their protective role and contribution to desirable properties such as viscosity, texture, and so on [11]. Different studies have demonstrated the advantages of including inulin, fructooliosaccharides, and galactooligosaccharides in fruit- and vegetable-based beverages [12].
As well as the viability of probiotics in fruit beverages, consumer acceptance of the product must be considered. It has been reported that probiotic inclusion changes the sensory properties of fruit juices depending on the particular strain, fruit, and storage temperatures that are used. Evaluations of consumer responses to a conventional fruit juice and a probiotic juice, found that most preferred the conventional juice, reporting “lactic” and “dirty” flavors for probiotic juice [13].
The aim of this research was to select the most acid-resistant and stable strain from three commercial probiotic bacteria to produce a mango beverage with acceptable functional characteristics. The impacts of its inclusion on the sensory properties of the beverages were evaluated during storage at 4 °C.
2. Materials and methods
2.1. Probiotic strains
Strains were obtained from commercial providers. L. rhamnosus HN001 and L. paracasei were obtained from Dupont-Danisco and L. casei from Mediterranea Biotecnologie. Strains were activated in 5 mL of De Man, Rogosa and Sharpe (MRS) broth and preserved in cryovials containing 30% glycerol at −20 ºC.
2.2. Acid tolerance test
Commercial probiotic strains were hydrated in 5 mL of MRS broth at 37 °C for 48 h. The microbial cultures were transferred into MRS agar with an inoculating loop. Colonies were harvested after the incubation period and the cell density adjusted using McFarland turbidity standard. The acid tolerance was measured in 5 mL of adjusted MRS broth with HCl 3N at different pH values (3.0, 3.5, 4.0, 4.5 and 6.0) after 0, 2, 4, and 24 hours of incubation [14].
2.3. Viability of probiotic strains in mango juice
Activated L. rhamnosus HN001 and L. paracasei were cultured for 12 h and L. casei was cultured for 20 h in MRS broth at 37 °C. The cultures were centrifuged at 4500 rpm for 15 min. The pellet was washed twice with sterile saline solution (0.85%) and then suspended in 10 mL saline. 4 mL (2 % vol/vol) of cell suspension was used to inoculated 200 mL of MRS broth (control). For the development of mango beverages, strains were cultured in a synthetic mango medium (MRS broth plus 10% fruit juice). Lactobacillus paracasei and L. rhamnosus were cultured in the medium for 24 h and L. casei for 30 h at 37 °C [15]. The inoculated MRS broth and mango juice were stored at 4 °C for up to 3 weeks. Samples to measure viability were taken every 4 days. Probiotic viability (CFU/mL) was determined by plate count using MRS agar after culturing at 37 °C for 72 h [16]. Mango beverages were prepared in the fruit and vegetable processing plant of the Instituto de Ciencia y Tecnología de Alimentos (ICTA) of the Universidad Nacional de Colombia following good manufacturing practices using mangoes of the Tommy Atkins variety. Sterilization of the beverages was performed at 121 °C, 15 psi for 15 min prior to inoculation with probiotic strains.
2.4. Effect of the prebiotic on probiotic growth
The strain that was most stable in the mango beverage was used to assess the effect of prebiotic substances on the growth of probiotics. Assays were carried out as follows in 30 mL MRS broth (supplemented with sucrose to simulate juice formulation) and mango beverage with 1 or 5 % (w/v) of inulin or FOS [17]. The inoculum was prepared in MRS broth and synthetic medium as described before. Cultures were incubated at 37 °C for 54 h, and growth measurements were carried out at 0, 24, 48, and 54 h.
2.5. Effect of storage at 4°C on viability, physicochemical and sensory stability of the probiotic mango beverage
The viability, physicochemical, and sensory characteristics were evaluated after the most stable strain and prebiotic were selected and included in the mango were beverage. A 22 factorial design (four treatments) was used to evaluate the effect of probiotic inclusion. Sampling was carried out every 7 days for 4 weeks. The treatments where defined as follows: treatment 1 (T1) corresponded to mango beverage, treatment 2 (T2) mango beverage + probiotic, treatment 3 (T3) mango beverage + prebiotic, and treatment 4 (T4) mango beverage + probiotic + prebiotic. Each characteristic was measured in duplicate for each treatment.
2.5.1. Probiotic strain viability in mango beverage supplemented with FOS at 5%
The probiotic viability for T2 and T4 was measured as described in section 2.3.
2.5.2. Physicochemical stability
pH measurements were carried out using a Metrohm® pH-meter (Suizo) according to AOAC 981.12 (AOAC, 2012). The total soluble solids (TSS) were measured as the degrees of Brix at 20 °C using an Atago® refractometer (AOAC 932.12). For titratable acidity, 5 g of beverage were dissolved in 10 mL of distilled water. The solution was titrated with 0.1N NaOH AOAC 935.57 (AOAC, 2012). Color (L*-luminosity, a*-red/green and b*-yellow/blue) was assessed using a CM.5 Konica Minolta spectrophotometer. The change in color (ΔE) was calculated using Eq. 1 [18]:
Sugar consumption (sucrose, glucose, and fructose) was determined by high performance liquid chromatography (HPLC) using a system with a PU-2031 bomb, CO-2065 oven, refractive index RI-2031 plus and a sugar-Pak I 10µm, 6.5 mm × 300 mm column (Jasco Inc, Italy) equipped. Samples were prepared by diluting 5 g of beverage in 50 mL of HPLC-grade water and centrifuging at 5000 rpm for 10 min. Supernatant was filtered in a hydrophilic sterile filter (0.45µm SARTORIUS) and C-18 Sep-Pak cartridge. For sucrose, fructose, and glucose measurements, a phenomenex REZEXTM RCM-monosaccharide Ca2+ column was used (methodology adapted from AOAC 2979.23).
2.5.3. Sensorial stability of the probiotic mango beverage
Sensory analysis was performed by 60 untrained panel members using samples of T4. The consumer acceptance test was carried out using a nine-point hedonic scale. Significant differences in acceptance between samples stored in different conditions were estimated using Friedman’s test. Six samples were taken during the 4 weeks of storage at 4 °C using reversed design, which allowed evaluation of all samples in just 1 day [19].
3. Results and discussion
3.1. Acid tolerance
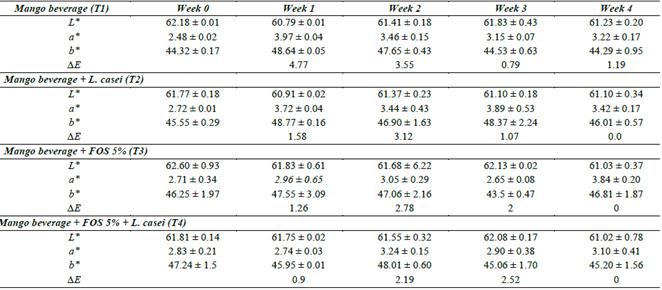
To simulate the acidic conditions of fruit beverages, five pH values between 3.0 and 4.5 were chosen, and their effects on probiotic viability are illustrated in Fig. 1. Significant differences in the strains’ tolerance of acid environments were observed at various time points of evaluation (p < 0.05). The most resistant strain appeared to be L. casei, as the bacterial population was not significantly different at pH 4.5 or 4.0 compared with the control (plain MRS broth). However, the cell concentration did decrease by 1.9 Log CFU/mL at pH 3.0. In contrast, L. rhamnosus showed lower resistance to acid, as changes in pH resulted in a lower cell density than the control. The most sensitive strain was L. paracasei. Within 2 h of incubation the viability of cultured cells at pH 3.0 was zero. All three strains showed the highest viabilities at pH 4.0 and 4.5.
Source: Author
Figure 1 Acid tolerance in MRS broth of (A) L. paracasei, (B) L. rhamnosus and (C) L. casei. (▲) MRS no modified, (■) pH 4.5, (○) pH 4.0, (◊) pH 3.5 and (●) pH 3.0.
These results are useful for the development of a probiotic carrier beverage which is produced under a similar pH range (3.0-4.5). Corcoran et al. (2004) reported that pH resistance depends on the strain used and glucose content in the culture medium [4]. These authors reported that L. rhamnosus GG had a higher viability at pH 2.0 in simulated gastric juices with glucose than either L. paracasei (NFBC 338 2.0), L. salivarious (UCC 500), or L. gasseria (ATCC 3323). However, when the sugar was removed from culture medium, L. gasseria was the most acid-resistant strain with a cellular concentration of 8.84-7.71 Log CFU/mL after 45 min at 37 °C. The populations of the other strains decreased by around 6-8 logarithmic units in these conditions.
Comparing the results of Corcoran et al. (2004) with the acid tolerance assays presented in this research, it is important to note that MRS broth is rich in glucose. This could have had a protective effect on strains by providing an energy source and metabolic precursors for pH homeostasis [20]. In the mango beverage, the acid resistance may change due to the composition and concentration of sugars. Nonetheless, the characterization of strains in MRS broth with modified pH levels is a useful tool for strain technological performance in probiotic food [3].
The above results are in line with other studies of the acid resistance of L. casei. For example, Wu et al. (2012) evaluated the physiological heterogeneity of this species in fermentations of residual yogurt whey under controlled and uncontrolled conditions (pH 6.5 and pH < 3.5, respectively, for 16 h at 37 °C) [21]. The authors found that the percentage of metabolically active cells was higher in uncontrolled pH conditions than in controlled conditions. This indicates that L. casei has developed protective and repair mechanisms, as well as systems for pH homeostasis such as F1F0 ATP synthase, arginine deaminase (ADI), and the glutamate decarboxylase system (GAD) [22].
3.2. Viability of probiotic strains in the mango beverage
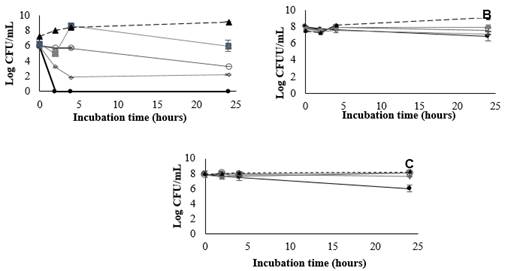
The storage temperature has an effect on the viability of probiotic bacteria when they are included in vegetable and dairy matrices [23]. For this reason, most studies of fruit beverages evaluate the probiotic behavior during storage at 4 °C. The bacterial population dynamics in MRS broth and mango juice at 4 °C during 20 days of storage are shown in Fig. 2A. For MRS broth, the cell densities of L. casei and L. rhamnosus stayed around 8 Log CFU/mL throughout the analysis period, with some population variation observed for L. casei (p < 0, 05). After 4 days, the L. paracasei population had decreased by 1.47 Log CFU/mL, despite the higher initial cell numbers compared with the other two species. However, over the next 15 days the cell density stayed constant at 8 Log CFU/mL.
Source: Author
Figure 2 Viability probiotic strains in (A) MRS broth and (B) mango beverage. (◊) L. paracasei, (■) L. rhamnosus and (•) L. casei.
The ability to survive in the mango juice matrix is shown in Fig. 2B. The results suggest that L. rhamnosus started to lose viability after 15 days of cold storage. Despite the population fluctuations during analysis, the initial cell densities of L. casei and L. paracasei were not significantly different to the densities at day twenty (p = 0.812), meaning there was no loss of viability. In contrast to its viability in MRS broth, L. paracasei was more stable than L. rhamnosus in the mango beverage. Fluctuations of L. casei and L. paracasei cell densities during storage could be related to the development of dormant physiological states due to conditions of environmental stress. A dormant cell is viable but not culturable, meaning that it is metabolically active but not capable of cell division or forming a colony on an agar plate [24]. It has been demonstrated that probiotics might become dormant during storage in an oat beverage by comparing plate count methodology with other quantitative analyses such as fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), and LIVE/DEAD Backlight kit [25].
Comparing the cellular densities of each strain in MRS and mango beverage at 4 °C, L. casei showed the highest stability as it resisted low pH levels and the population stayed constant in refrigeration conditions. In general, the selection of probiotic strains is based on acid tolerance tests in the matrix that they will be included in during storage. Although a strain may be capable of resisting stomach acidity for around 2-4 h, it may not resist the acidity of the product during extended storage, because intracellular protons accumulate with time causing structural, DNA, and protein damage [26]. For this reason, L. paracasei and L. rhamnosus were not selected for inclusion in a probiotic mango beverage.
3.3. Effect of prebiotic inclusion on probiotic growth
According to the results of the analysis of bacterial population dynamics in acid and cold storage, the most stable strain to include in the mango beverage was concluded to be L. casei. Thus, L. casei was chosen for evaluation of the effect of prebiotics on growth in two different media: MRS broth and the mango beverage. The results (Fig. 3) revealed very different growth behaviors between the media. Cell density after 24 and 48 h was higher in MRS and MRS + 5% sucrose than that observed when prebiotics were included. However, positive effects of the inclusion of inulin and FOS were observed at the end of the assay period (54 h), and there were clear differences (as analyzed by Tukey’s test) between the controls and prebiotic treatments. These results suggest that FOS and inulin could be included in beverages at either of the concentrations tested. The growth of L. casei in the mango beverage was significantly higher when 5% FOS was included (Fig. 3B), indicated by consistently higher cell densities during the 48 h of incubation.
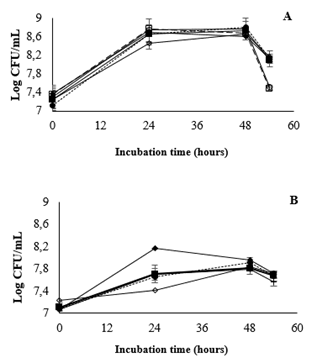
Source: Author
Figure 3 Prebiotics growth effect on L. casei at 37°C in (A) MRS broth and (B) mango beverage at 37°C. (-○-) MRS, (--☐--) MRS + 3% sucrose, (-■-) FOS 1%, (-♦-) FOS 5%, (··•··) Inulin 1%, (-◊-) Inulin 5%, (-+-) mango beverage.
Overall, there were no significant differences in bacterial growth between the prebiotic and control treatments at the end of the assay period. Therefore, MRS broth is a useful tool for examining the effect of prebiotic inclusion on bacterial growth, but the selection process must be carried out in the matrix beverage. The inclusion of 5% FOS in mango juice can be used to protect cells during cold storage. Adebola et al. (2014) reported similar results as observed in this study; a low polymerization grade prebiotic promoted high growth rates of L. acidophilus NCFM and L. reuteri (NCIMB 11951), while inulin, despite its wide use in food formulations, was a less effective carbon source[27]. Likewise, Kaplan and Hutkins (2000) studied L. plantarum growth in MRS basal broth supplemented with different polymerization grade FOS (GF2, GF3, and GF4; GF = glucose monomer linked to fructosyl units). They observed that the fermentation rate was higher in GF2 and GF3 than GF4 [28]. In 2003, the same authors determined the system involved in L. paracasei 1195 in vitro oligofructose hydrolysis. It was found that the bacteria uptook transformed FOS via an ATP transport system, but were unable to effectively metabolize inulin despite having the same chemical structure as FOS [29].
In view of the above, 5% FOS had the greatest effect on the growth of L. casei, and was chosen to supplement the mango beverage for the physicochemical and sensory assays. This reduced the possibility of pH-induced cellular damage as it may provide a substrate for metabolism and a physical barrier against environmental stress [30].
3.3. Effect of storage at 4 °C on viability, physicochemical, and sensory stability of probiotic mango beverage
3.3.1. Viability of probiotics in the mango beverage supplemented with 5% FOS
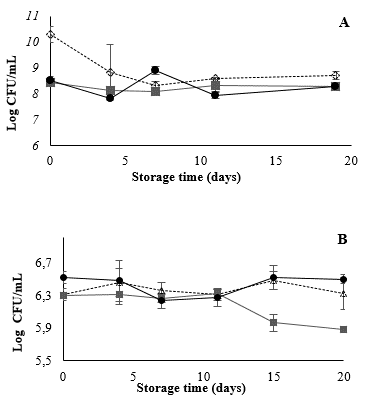
Prebiotic assays showed that 5% FOS positively influenced the growth of L. casei in MRS at 37 °C. However, Fig. 4 shows that the L. casei populations in the mango beverage were the same throughout storage at 4 °C regardless of FOS inclusion (7 Log CFU/mL) (p > 0.05). These results are in line with those obtained in previous tests, which indicated that the mango matrix would be an ideal carrier of probiotic microorganisms despite its acidity and antimicrobial components (such as phenolic compounds) [31]. Temperature can have negative effects on viability, but in this case, 4 °C contributed to the population stability in conjunction with being favorable conditions for juice storage [32]. The population stability of L. casei in the mango beverage could be related to the positive effect of components such as ascorbic acid. This vitamin has the capacity to capture dissolved oxygen (a cause of low probiotic viability in beverages), generating an anaerobic environment by reducing the redox potential of the system. Ascorbic acid is, in fact, used as an additive in probiotic-supplemented dairy food to avoid cellular damage [5], and is used as a cryoprotective substance for microorganism storage [33].
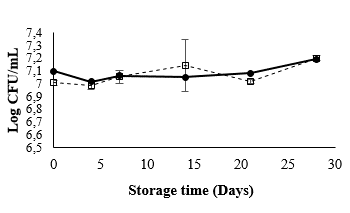
Source: Author
Figure 4 Viability L. casei in mango beverage during storage at 4°C. (--☐--) Mango beverage + L. casei, (-•-), mango beverage + L. casei + FOS 5%.
Because the mango beverage is an ideal carrier for L. casei, it should not be necessary to add FOS for storage at 4 °C. However, inclusion of this prebiotic would help the strain to survive in the gastrointestinal tract [11].
3.3.2. Physicochemical stability
Once the viability of L. casei in the mango beverage at 4 °C was ensured, the effects of its inclusion on the physicochemical characteristics of the beverage were estimated. The measured pH levels and titratable acidities for all treatments are shown in Fig. 5. Significant decreases in the pH of T2 and T4 were observed (p < 0.05); the pH of T2 dropped from 4.07 to 3.88, and that of T4 dropped from 4.07 to 3.77 across the 4 weeks of storage. The T2 and T4 samples showed titratable acid levels that were 24-27% higher than both the fresh beverage (at 0 weeks of storage) and the treatment in which L. casei was not added. Comparing these results with those of Kumar et al. (2015) [34], at different temperature, when L. plantarum was included in a mango beverage, the pH decreased from and the acidity increased at 37 °C. These differences could be explained by the storage temperature used in this study (4 °C), which may slow the acidification process and prevent the L. casei population increasing above 7 Log CFU/mL. The L. casei is considered a facultative heterofermentative bacteria. It produces lactic acid from, and can metabolize pentoses producing lactic and acetic acids, fermentation of hexose via the glycolysis pathway, ethanol, and CO2 [35]. Changes caused by the production of lactic metabolites and acetic acid depend on the strain and sugar metabolism [18]. Our study indicates that changes in the production of organic acids can occur regardless of the temperature, that is supported by studies that have demonstrated that some strains are capable of fermenting sugars in beverages during storage at 4 °C. It has been reported that the inclusion of L. paracasei ssp. paracasei in apple juice increased the acidity during storage, as the pH decreased slightly [30]. They hypothesized that the results were due to the presence of oligofructose and sugars, which resulted in the production of small amounts of organic acids [30]. Another explanation for the changes in pH and titratable acidity could be the buffering capacity of the mango beverage. Other authors reported total soluble solids changes and pH decrement after 7 days of storage returning to the original level after 14 days, suggesting that the juice has a high buffering capacity [36]. In the present study, the pH did not return to 4.07 and the concentration of dissociation of protons led to significant increases in the pH which intensified with time. Therefore, the buffering capacity of the mango beverage is lower than that of orange juice [36].
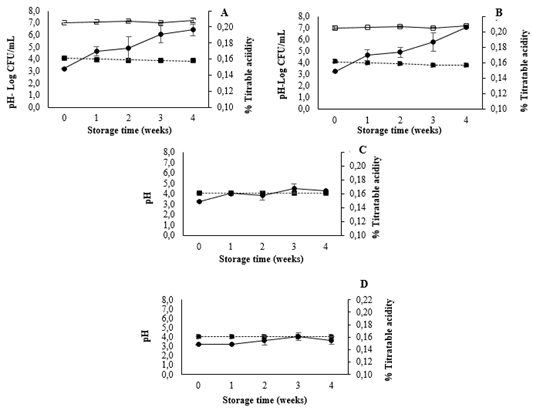
Source: Author
Figure 5 Effect of L. casei inclusion on the pH and titratable acidity of the mango beverage for (A) T2 (mango beverage + L. casei), (B) T4 (mango beverage + L. casei + 5% FOS), (C) T1 (mango beverage), and (D) T3 (mango beverage + 5% FOS). Key: (-☐-) L. casei population, (-●-) titratable acidity, and (■) pH.
Although large variations in pH and titratable acidity are not desirable in probiotic fruit beverages from a sensory point of view, the production of acid lactic by L. casei could help to avoid spoilage [37].
In contrast to the pH and titratable acidity levels, the total soluble solids and sugar contents (glucose, fructose, and sucrose) did not change significantly for any of the treatments during storage (Fig. 6). The differences between the initial concentrations of each sugar are due to the FOS content as well as the partial hydrolysis of sugar during sterilization [38]. It could be inferred that there was no hexose consumption or sucrose hydrolysis by L. casei under the evaluated conditions. However, when these results are combined with the pH and titratable acidity results in beverages supplemented with L. casei, the techniques used during this study were not able to detect consumption of any sugar component. It may also be the case that L. casei was functioning in a state of metabolic maintenance, which did not cause a drastic decrease in the total soluble solids or sugars.
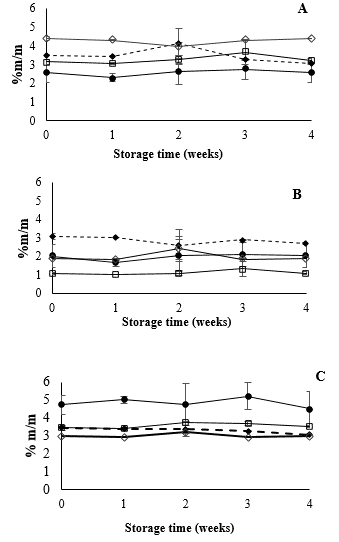
Source: Author
Figure 6 Effect of L. casei inclusion on mango beverages in sugar content for (A) fructose, (B) glucose and (C) sucrose. (-●-) T1-mango beverage, (--♦--) T2-mango beverage + L. casei, (-☐-) T3-mango beverage + FOS 5% and (-◊-) T4-mango beverage + L. casei.
Results of the color analyses are shown in Table 1. All treatments became darker, which could be a feature of the inoculum preparation in saline solution in T2 and T4, since the fresh sample showed lower L* values. In addition, increases in red (a*) and yellow (b*) colors were observed. The color changes during and at the end of the storage time were not above a ∆E value of 3.3, meaning that consumers would not be able to perceive them [39]. According to these results, the inclusion of L. casei and 5% FOS had no impact on color compared with control treatments. Although the statistical analysis indicates significant changes, these are not perceptible to the human eye.
3.3.3. Sensorial stability of the probiotic mango beverage
The results of the Friedman test graphics for consumer acceptance are shown in Fig. 7. The black and grey lines in the graphic representation of the confidence intervals for each analysis point. The acceptance of the beverage between day 0 of storage (red line) showed a significant difference (p < 0.05) to the acceptance at any other time point. The acceptance changed from a value of seven (like moderately) at 0 weeks to six (like slightly) by 1 week, and remained constant thereafter until the end of the fourth week in storage. This change may be caused by acidification of the mango beverage due to the inclusion of L. casei. Despite the change in consumer acceptance, off flavors in probiotic mango beverage were not reported. This may be because the sweetness and exotic components of mango such as alcohols, esters, lactones, acetoin, carboxylic acids, furans, and linalool oxide help to mask off flavors [31].
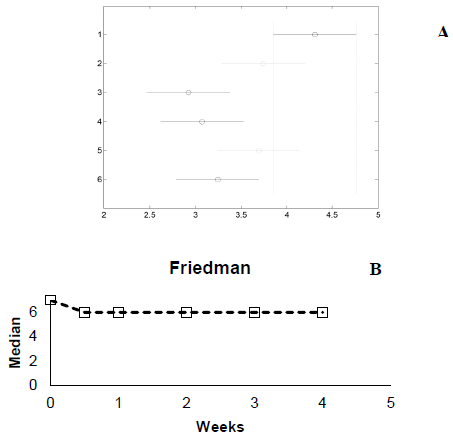
Source: Author
Figure 7 Friedman test consumer acceptance. (A)-confidence intervals, (B)-median graphic.
The inclusion of probiotics produces variations in physicochemical characteristics in a wide range of food matrixes. With this in mind, the addition of aromatic compounds and microencapsulated probiotics has been used as a technological strategy to improve the sensory and physicochemical stabilities in probiotic-supplemented juices [40]. Sohail et al. (2012) reported that the pH of orange juice supplemented with L. rhamnosus GG decreased pH from 3.8 to 3.52 [41]. Meanwhile, when cells were encapsulated in alginate (10-40 µm), the pH decreased to 3.62. Likewise, other studies have reported that the TSS and pH values were higher when microencapsulated cells were added to juice, compared with apple juice which included probiotics as free cells [42]. Another way of improving the acceptance probiotic beverages is to inform consumers about the health benefits of probiotics. For example, the acceptance of a probiotic-supplemented fermented cupuassu (Theobroma grandiflorum) beverage was greater when consumers were informed about the nutritional and health [37].
4. Conclusion
From the results of the acid tolerance and bacterial population assays in MRS broth and mango beverage at 4 °C, it can be concluded that the most suitable strain to use in the production of a mango beverage is L. casei. This matrix is suitable for the incorporation of the strain due to the fiber and carbohydrate content, which ensure a stable bacterial cell density of around 7 Log CFU/mL. The fiber and carbohydrate content of mango have previously been identified as beneficial to microorganisms during cold storage. A major challenge of adding probiotic microorganisms to fruit beverages is the impact on sensory attributes as a result of bacterial metabolism. Although pH and titratable acidity changes were observed in the mango beverage supplemented with L. casei, the product was accepted by consumers at all time points of the storage at 4 °C. This acceptance could be attributed to the exotic components and sweetness of mango, and the acid balance that helped to lower the impact of L. casei metabolites and mask off flavors.