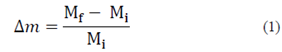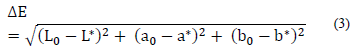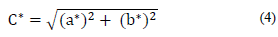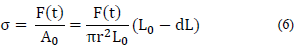1. Introduction
Currently, vegetable, fruit, root, and tuber consumption is moving from fresh to processed products with higher added value. Frozen products are of greater importance, especially in developed countries.
Sweet potato (Ipomoea batatas L.) is one of the world's most important food crops, ranking third, after potato and cassava in the production of roots and tubers, with more than 110 million metric tons produced per year. This important crop can help to combat food insecurity and malnutrition, both in underdeveloped and developing countries, because of its high energy content, as it is rich in carbohydrates, especially in starch, and because of its high concentration of β-carotene in the orange flesh sweet potato (OFSP), a precursor of vitamin A [31]. Sweet potato can be included as a main ingredient or as a substitute in several food products. Their use in the food industry is in the form of purees, flours, and starches, which are used as functional ingredients in various processed products. Sweet potato purees can be made into food products such as beverages, soups, baby foods, ice cream, baked products, breakfast cereals, and various snacks and desserts [8,29].
Technologies developed for the processing of purees are mainly based on cooking sweet potato roots (cut into slices, strips, and cubes), grinding or macerating them to obtain the puree that can either be used immediately, or preserved through refrigeration or freezing, and finally packaged under aseptic conditions for marketing [29]. Post-harvest handling of roots and the type of thermal treatment employed can have significant effect on sweet potato purees. Changes in carbohydrate components and enzyme activities may affect the appearance, texture, flavor, and nutrient composition thereof [25].
The aim of this study was to develop a high-quality puree from OFSP that could be used as a high-value nutritional ingredient in food products or for direct consumption. It was therefore essential to investigate the effect of thermal pre-treatments, such as blanching and freezing, on the physical properties of the OFSP variety with high β-carotene content.
2. Materials and methods
2.1. Material and sample preparation
OFSP roots from the CIP-440287 cultivar with high β-carotene content (250-300 μg/g DM), were grown in the field at the International Center for Tropical Agriculture (CIAT), Valle del Cauca, Colombia, and harvested after 4 months. Roots were selected based on commercial consumer preferences for shape, size, and weight. Sweet potato roots with external damage (phytosanitary damage or symptoms of disease or pest infestation) were removed. Selected roots were washed with tap water, peeled manually, and were cut into cylindrical samples (ca. 1-in diameter x 15-mm length) from the central part of the roots.
2.2. Thermal treatments
Thermal treatments were carried out in accordance with the following processes:
2.3. Blanching and freezing/thawing processes
Raw sweet potato samples were blanched in de-ionized water at 95 °C for 2 min. Heating plates (Thermo Scientific, USA) were employed, using glass beakers with a raw material/water mass ratio of 1:10. Both blanched and unblanched material was frozen at -80 °C for 40 min, so that their geometric center reached -40 °C, in an ultra-low freezer (Thermo Fisher Scientific ULT2586-9SI-A38, USA). Therefore, a rapid freezing rate (approximately 2 °C/min) was used to promote the formation and distribution of small ice crystals, and to minimize tissue damage and drip loss during thawing. Frozen material was stored at -18 °C for 1-72 days in a home freezer (Electrolux FE26, Colombia) prior to cooking. The cooking process was conducted in glass beakers on heating plates (Thermo Scientific, USA) at 95 °C for periods of between 1-24 min. A frozen material/deionized water mass ratio of 1:10 was used.
As a control sample, only the cooking process was carried out at 95 °C for 15 min (normal cooking time is 15 min). For the analysis, frozen samples were thawed at 8 °C for 18 hours in a home refrigerator (Whirlpool Company, Italy) in order to avoid and prevent the icing damage.
2.4. Determination of physical properties
2.4.1. Drip loss (Δm)
The amount of drip loss, after thawing samples, was assessed by weight, measuring that of each sample before and after freezing/thawing. Drip loss or liquid phase loss was determined using an analytical balance (Ohaus, EUA), and was calculated based on eq. (1), expressed as a fraction.
Where Mi is the weight of the sample before freezing, and Mf is the weight of the thawed sample.
2.4.2. Volume change (ΔV)
The volume variation in each sample was determined by measuring its diameter and height, before freezing and after thawing. The measurements were made in triplicate by taking three points at 120° of the cylinder circumference, using a Vernier caliper. The volume change was calculated using eq. (2).
Where Vi is the volume of the sample before freezing, and Vf is the volume of the thawed sample.
2.4.3. Color measurement
OFSP sample color values of were determined using a digital chroma meter (Konica Minolta CR-410, Japan) in the CIELAB system. The color was described based on the L*, a* and b* values, where L* is a measure of lightness, a* defines components on the red-green axis, and b* defines components on the yellow-blue axis. Standard illuminant C was used as the light source. The equipment was calibrated before each measurement using a calibration plate. The measurements were made five times for fresh, treated, and control samples.
The color indicators, relative total color difference ΔE, chroma C*, and hue angle h were calculated with the use of eq. (3)-(5) [19].
2.4.4. Texture measurement
Textural properties were determined using a food texture analyzer (Stable Micro Systems TAxT2, UK) with built-in Texture Expert Exceed software. Two tests, viz., the uniaxial compression test and the shear/cutting test were performed with a 25 kg load cell capacity. The texture of fresh, treated, and control OFSP samples was measured at a test speed of 1 mm/s to 20% of its original height, using a 40 mm aluminum cylindrical probe (SMS P/40), and to 50% of its original height using a craft knife (HDP/BSK blade set with knife), for compression and cutting/shearing respectively.
The stiffness or modulus of elasticity Ed (kPa) was calculated from the stress-strain curves, as the slope of the initial straight-line portion of the curve. Stress-strain compression curves were constructed from the force and deformation data, using eq. (6)-(7) [5].
Where σ is the stress, εH is the Hencky strain, F(t) is the momentary force, A0 is the initial cross-sectional area, r is the radius of the sample, L0 is the height of the unstrained sample, and dL is the deformation rate.
In the cutting/shearing experiments, the maximum peak force obtained from the graph was taken as firmness F (gf) [16]. Five measurements were performed for each treatment, and the results were expressed as the average.
3. Experimental design and statistical analysis
The effect of the cooking time and freezing storage time on the quality of fresh, only cooked, blanched, and unblanched OFSP was studied using Response Surface Methodology (RSM). A central composite rotatable design was used for cooking time, which ranged between 1 and 24 min, and with freezing storage time ranging between 1 and 72 days, as independent variables. The design required 13 variable combinations that were performed in random order, including five replicates of the center region to generate a quadratic response surface (Table 1). In both processes, each treatment combination was repeated twice, and all results were averaged. Range finding experiments were performed at the outset of this study, in order to ascertain what cooking temperature and time ranges could be applied to the OFSP, such that the product would be acceptable to consumers.
All experimental results were statistically analyzed, using statistical package Minitab 16 (version 16.2.4; Minitab Inc., USA) to perform one-way analysis of variance (ANOVA) and Tukey’s significant multiple comparison test at a 95% confidence level (α = 0.05). Differences were considered to be significant at P < 0.05.
4. Results and discussion
4.1. Drip loss and volume changes
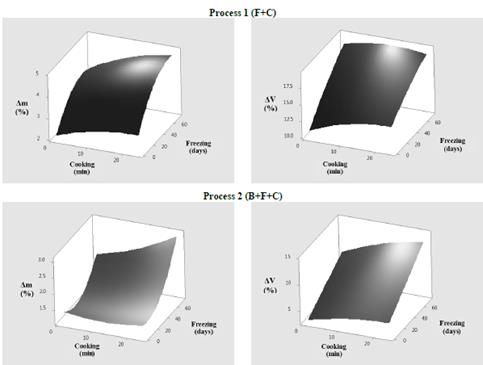
The results of physical changes such as drip loss (Δm) and volume variation (ΔV) during OFSP sample frozen storage, submitted to the different evaluation processes, are shown in Fig. 1.
Source: author elaboration.
Figure 1 Drip losses (Δm) and volume variations (ΔV) of blanched and unblanched OFSP samples.
According to these results, at higher cooking and freezing times, an increase of Δm in Process 1 (F+C) of about 5% was observed in samples frozen for longer than 72 days. In Process 2 (B+F+C), there was less drip loss (<3%) in the previously blanched samples with the same freezing time. Starch granules formed by amylose and amylopectin in a cold slurry tend to swell, retaining water, and at a certain temperature the starch, gelatinizes, thickening the liquid. When this gel is at rest, the linear chains of amylose are bonded as if they crystallize and release part of the water previously retained in its structure, in a process called syneresis. Thus, the negative effect of freezing time is related to ice crystal growth, caused by fluctuations such as time increases. This could cause the rupture of the fluid cellular structure and result in greater drip loss [1]. The increase in drip loss during frozen storage time was similar to that reported by [18], who evaluated the stability of frozen Mexican tubers, presenting greater syneresis with longer storage times in makal, sago, sweet potato, and cassava starches.
On the other hand, the lower drip loss of the blanched samples may be associated with starch pre-gelatinization, and thus with water retention capacity increase in the gel structure. Unblanched samples presented low stability in freezing/thawing, which caused greater loss of the water trapped in the gel. In addition, although blanching prior to freezing exerts an effect on the starch granules and pectic substances in frozen tissue, with a faster freezing rate the cells and the intercellular spaces are relatively small, which may also explain the firmer texture of frozen products, when compared to those without pre-treatment. A similar behavior was observed in cassava, sweet potato, and yam, with respect to the drip loss [26].
Regarding ΔV, a volume decrease was observed in OFSP samples for all processes evaluated at higher freezing times. Maximum values of ΔV were obtained, independently of cooking time, in freezing times in excess of 72 days, in frozen-and-cooked samples with a volume variation of 21%, while blanched-frozen-and-cooked samples presented 13% volume variation. This behavior in the volume decrease is associated with the increase in drip loss during freezing, due to the ice recrystallization process. According to [6], drip losses in food produce damage to the structure, causing shrinkage and microstructural changes.
Root tissues, such as sweet potato, have semi-rigid cell structures, and the structure of their cell wall is less elastic than the cell membrane, thus these tissues show less resistance to the expansion of ice crystals by volume. As such, they are exposed to ice crystal damage during freezing. However, with blanching as pre-treatment and a high freezing rate, the intersection of heterogeneous nucleation and the growth of ice crystals results in the formation of numerous small intracellular crystals, which vary in size, inversely to the number of nuclei. This produces a minimum migration of water to the crystallization sites, which causes less mechanical damage in the product cellular structure, and consequently smaller volume loss [10,14].
4.2. Color differences
OFSP sample color parameters for the evaluated processes are given in Table 2.
Table 2 Color parameters of fresh, control, blanched, and unblanched OFSP samples after freezing (72 days).
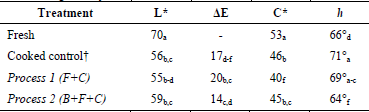
†Unblanched and cooked samples (15 min).
Different letters in the same column indicate significant differences.
Source: author elaboration.
Lightness (L*) of fresh OFSP presented a value of 70, and after 15 min of cooking (control treatment), this decreased to 56. Comparing these values with the results of the evaluated processes, an increase of L* was observed in Process 2 (B+F+C), indicating a control sample that was darker than blanched-frozen-and-cooked samples. In contrast, L* was decreased in Process 1 (F+C), which indicates frozen-and-cooked samples which were darker than the control sample. Therefore, the application of blanching as pre-treatment to Process 1 (F+C) resulted in OFSP samples with a lighter color. This shows the positive effect that blanching has, as it diminishes the differences in lightness between the cooked and frozen-and-cooked samples. The darker color of frozen-and-cooked products might be associated with mechanical damage (ice crystals and volume expansion) caused by the freezing/thawing processes, as they may disintegrate the fragile chlorophyll and carotenoid membranes, thereby facilitating their oxidative or enzymatic degradation [13]. A similar behavior was observed in peach puree [4] and mashed potato subjected to the blanching and freezing/thawing processes [1].
Regarding total color difference (ΔE), was lower in Process 2 (B+F+C) and higher in Process 1 (F+C), compared to the control treatment in which the ΔE was 17 (after cooking for 15 min). The comparison of the estimated color differences, with respect to the control sample, shows that blanching prior to freezing affects sample color less. This result coincides with that obtained in the lightness analysis, confirming that the freezing/thawing of product had a decisive effect on the final OFSP color. Process 1 (F+C) presented the highest ΔE values, compared to Process 2 (B+F+C) or to the corresponding control. Color alterations during storage of frozen vegetables as a result of enzymatic browning are caused by the oxidation of phenols in the presence of oxygen. Other authors have reported that blanching prior to the freezing of sweet potato had a positive effect on the prevention of enzymatic browning caused by the oxidation of phenols, which significantly reduced polyphenol oxidase (PPO) activity [15,21].
Chroma is a measure of the saturation, vividness, or intensity of color, and is completely separate from hue and lightness. For the control treatment, C* decreased after 15 min of cooking from 53 to 46. This decrease in chroma indicates a loss in color intensity in OFSP samples, denoting a less saturated, duller color after cooking, which might be an indicator of browning. Similar behavior was observed in tomato, as consequence of the carotenoids pigments degradation, caused by exposure to high temperatures, light, oxygen, and pH changes [3]. A minor decrease of C* in Process 2 (B+F+C) was also observed, which indicates a saturated color in OFSP samples. On the contrary, in Process 1 (F+C), the chroma presented a greater decrease than the control treatment. Similarly, samples that were blanched prior to freezing showed an increase in chroma, as compared to samples without pretreatment. This result emphasizes the potential that a preliminary blanching can have for the freezing and cooking processes to increase color purity. The beneficial effect of blanching as pretreatment, on the chroma differences, was similar to that observed in freeze-dried pumpkin [12].
In this study, the hue angle h orients the proportion of reds and yellows in the fresh and treated samples. For the control treatment, h was increased from 66° in the fresh product to 71° in the cooked product after 15 min. Compared to the results of the two processes, lower h values were observed in both blanched and unblanched OFSP samples. Hue reduction may be attributed to a loss of carotenoids, located superficially in the pulp due to the effects of temperature, acidification, and/or oxidation. Moreover, it should be considered that the cooking temperature modifies the cellular membranes and cell wall, which permits greater carotene extraction from the cooked pulp [22].
In general terms, the different processes evaluated in OFSP samples show a reduction in lightness L* and chroma C* values. However, they also reflect an increase in the value of hue angle h in the treated samples, compared to fresh samples. These color differences in the sweet potato during thermal processing may be associated with various chemical reactions, such as carotenoids isomerization and oxidation, anthocyanin degradation, sugars caramelization, and enzymatic/non-enzymatic browning reactions [20]. Similar results were reported in sweet potato [23,32,34], pumpkin [12], and potato processing [1].
4.3. Texture analysis
The results showed that, in the processing situations considered (frozen-and-cooked, blanched-frozen-and-cooked), cooking time and freezing storage time had a significant effect on the mechanical properties of OFSP, derived from the uniaxial compression and shear/cutting tests. The results of the texture parameters (modulus of elasticity and firmness) for the two processes evaluated are shown in Fig. 2.
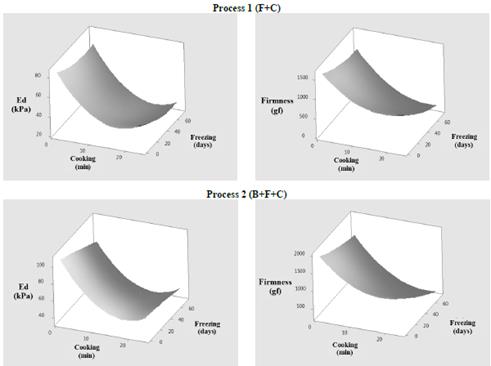
Source: author elaboration.
Figure 2 Texture properties: Elasticity modulus (Ed) and firmness (F) of blanched and unblanched OFSP samples.
The values for rheological parameters, elasticity modulus Ed and firmness F, which represent the mechanical response of the internal cellular pressure, decreased in all thermal processes evaluated, as compared to the fresh sample. Therefore, the internal cellular pressure, cell wall stress-strain relationship, and consequently the total stiffness of the tissue, are affected by the thermal treatments. The changes in sweet potato textural parameters are related to the transformations in starch properties, possibly with a high percentage of gelatinization, which is associated with greater water absorption, which in turn produces hydrolysis and cell wall solubilization [2,17,29].
It should also be noted that the textural properties of sweet potato and many other vegetables, roots, and tubers depend on the presence of pectic substances which form part of the intercellular material. This includes the pectin methylesterase (PME) enzyme, which is more easily soluble during processing than other cell wall polymers. This results in a decrease in intercellular adhesion, which subsequently contributes to texture degradation [17,30,33]
In general, changes in the texture of roots such as sweet potato depend on several factors: starch gelatinization, pectin degradation, cell wall breakdown, and cell separation. These phenomena translate into a marked decrease in tissue stiffness, hardness, and firmness.
In the results, Ed was decreased in all treatments, in Process 1 (F+C) at higher cooking and freezing times, while in Process 2 (B+F+C) Ed was decreased in samples which had been previously blanched with a longer freezing time (>40 days). Minimum Ed values were presented with cooking times greater than 15 min. For the control treatment, Ed decreased from 186 kPa (fresh product) to 64 kPa (cooked product for 15 min). Based on the control treatment result, the frozen-and-cooked samples and the blanched-frozen-and-cooked samples had lower values of Ed compared to the samples which had only been cooked. This softening appears to reflect superior damage from heat treatments in the tissue of OFSP samples. The decrease in Ed during thermal processing can be considered a manifestation of the mechanical response of the elasticity of cellular membranes or intercellular adhesion in the tissue, which is modified according to the magnitude of the degradation of the starch and cellular wall substances [9,27].
Analyzing the effects on the original structure of the product for each thermal treatment involved in the processes evaluated, as well as their combinations, Ed had a greater decrease in the frozen-and-cooked samples, and a lower decrease in the blanched-frozen-and-cooked samples. This behavior confirms the fact that freezing and cooking soften the tissue in very different ways: freezing causes cell wall rupture, whereas the cooking causes softening via cellular separation, without breakage, and leaving the structure of the cellular wall almost intact [1,29].
As starch is the predominant substance in sweet potato, variations in texture during cooking are mainly due to changes in this carbohydrate and in pectic substances. The gelatinization and retrogradation of starch and an enzymatic and non-enzymatic degradation of the pectin which constitutes the cell wall are produced. Moreover, the gelatinized starch structure is damaged in freezing due to the pressure exerted by the ice matrix on the granules. This tissue damage could lead to loss of cell membrane function, disturbance of metabolic systems, protein denaturation, permanent transfer of intracellular water to the extracellular medium, enzymatic inactivation, or wide cellular rupture [9].
In relation to OFSP cylinder firmness F (derived from shear/cutting tests), a similar behavior was observed in all evaluated processes, indicating a decrease in force in longer cooking and freezing times. Minimum values of F were presented in samples with cooking times of greater than 15 min and freezing times greater than 40 days. In the control treatment, F decreased from 5,407 gf in fresh samples to 804 gf in cooked samples. Compared to the control treatment result, a lower force was observed in frozen-and-cooked samples than in the sample which had only been cooked. These differences may be a consequence of the freezing process, which produces negative effects on the textural quality of roots, due mainly to the phenomena of crystallization. Even when freezing is performed at a rapid freezing rate, a certain degree of damage and cell wall rupture is inevitable. In addition, some cell wall materials damaged in the freezing process are lost during subsequent cooking, which justifies the lower firmness observed in frozen-and-cooked tissue compared to the control sample.
Another possible explanation for this behavior is that the structure of the gelatinized starch granule is damaged at freezing temperatures, due to the pressure exerted by the ice matrix on the granules. Gelatinized starch also presents greater retrogradation as a consequence of freezing/thawing [11]. As expected, when blanched tissue was frozen-and-cooked, the firmness decreased relative to that of tissue that was only cooked. However, the blanching pre-treatment produces an increase in texture compared to the unblanched samples, which may be associated with an increase in the mechanical resistance of OFSP tissue, which is especially notable after being subjected to additional processing operations.
It has been shown that the effect on the firmness of the pectin de-esterification, which is attributable to the pectin methylesterase (PME) enzyme, becomes evident only after heating at high temperatures, as in blanching or sterilization. De-esterified pectin is less susceptible to subsequent degradation, and is therefore more heat-stable. In this way, post-processing results in greater pectin insolubility, which is generally thought to increase cell-cell adhesion [24]. According to [28], sweet potato tissue blanched at a certain temperature for a certain period of time behaves as an elastic solid with a resistant and strong texture. However, other authors suggest that the firmness effect detected sweet potato boiled in water is not only caused by the PME enzyme, but also by the decomposition of starch into sugars that migrate from the cell without causing cell separation [7]. Still other studies point out that the contribution of starch to the texture and firmness of cooked potatoes predominates over the role played by changes in the constitutive polymers of the cell wall [30].
In products, where starch is the major constituent of dry matter, the establishment of which structural components and modifications thereof are responsible for tissue firmness when subjected to thermal treatments, is a matter of controversy among different researchers, often attributing to the hydration, swelling, gelatinization and retrogradation of the starch, stability of the cell wall, and pectic substances of the cell wall, or to a combination of the modifications of both structural elements.
5. Conclusions
OFSP processing by combining thermal treatments, such as blanching, freezing, and cooking, results in physical tissue changes. The freezing storage time (72 days) and subsequent thawing presented significant drip losses and volume changes in the OFSP cylinders, independently of the combined thermal process. Also, it was evident that the storage time during freezing showed greater influence in the physical OFSP changes than cooking time. Blanching as pre-treatment was positive for sweet potato quality loss reduction during freezing storage, as it decreased drip loss, volume change, color (less loss of lightness, color purity, hue angle, and total color difference, i.e. lighter and more intense color, than unblanched samples) and firmness losses.