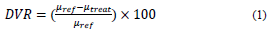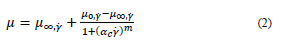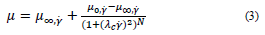1. Introduction
As the petroleum industry advanced over the last century, it encouraged many of the technological advances that developed over the same period, providing a cost-effective energy resource in almost all human social spheres [1]. However, as conventional crude oils have been exploited to satisfy world energy demands, HO (heavy oil) and EHO (extra-heavy oil) reserves have gained significant importance [2]. Nevertheless, exploiting HO and EHO carries appreciable technological challenges and issues for the petroleum industry in the different processes of production, transport, and refining due to the fluid’s properties, such as low API gravity, emulsifying tendencies, low gas/oil ratios, a high content of heavy components and high viscosities [3]. There is no direct relation between fluid density and viscosity in crude oils. However, it is common to find crude oils with low API gravity that have a high viscosity [4,5]. Oil mobility is a significant factor which accounts for fluid flow capacity through porous media and depends on relative permeability and fluid viscosity ratio, directly affecting recovery factors from a specified reservoir [6,7]. Also, at surface conditions, there are several standards for transporting fluids that include specific viscosity conditions [3]. HO and EHO high viscosity is caused by the large amount of organic compounds such as resins and asphaltenes that they contain [8,9]. Since HO and EHO have a high asphaltene and resin content, these compounds self-associate, leading to the formation of a complex viscoelastic network which is the main cause of high viscosity [10-13].
Several studies have been carried out to develop different kinds of treatments for increasing HO and EHO mobility at reservoir conditions (in-situ) and improving their properties for transport once brought to the surface. In-situ treatments include thermal enhanced oil recovery (EOR) [14-17], injection of solvents and light oils [18-20], deasphalting [21,22], and other treatments based on nanotechnology [7,23,24]. However, in many cases, these operations have been proven to promote distinct formation damage (FD) processes such as unwanted lithological changes related to mineral dissolution and transformation, wettability alterations, absolute permeability reductions due to the high complexity of the techniques and the completely unknown conditions in the reservoir which greatly encumber flow assurance [25].
At the surface, different techniques have been implemented, including heating [26,27], dilution [28], transport by oil-in-water (O/W) emulsions formation [29], partial upgrading [30], electromagnetic fields induction [31], and annular flow [32]. Nevertheless, these methods may have an adverse environmental impact [22,33] and could lead operations to be non-cost-effective due to high quantities of diluent, low perdurability, the possibility of formation damage due to the inestability of asphaltene in the presence of diluents such as naphta, enviromental impact due to low saturation pressure and high evaporation, or the additional costs of separation mainly in the emulsion formation [34].
Therefore, new and cost-effective technologies need to be developed to fulfill the production and transport requirements of HO and EHO. Hence, ultrasound application for the creation of cavitation bubbles has been proven to upgrade HO feedstock by breaking the heavy molecules followed by a hydrogenation process [35]. Cavitation has been proven to foment an increase in temperature and pressure as gas bubbles collapse within the working fluid [36,37]. It has been reported that the application of ultrasound cavitation along with surfactants can reduce the asphaltene content by 35% by converting the asphaltenes into gas oil and resins [36], using the water from an Oil-in-Water emulsion (O/W) as a hydrogen donor in order to generate termination reactions between asphaltene free radicals obtained by cracking reactions and the dissociated hydrogen radicals from the water. Likewise, Kaushik et al. [38] employed ultrasound cavitation for vacuum residue upgrading, generating positive physicochemical changes in the feedstock.
Nowadays, nanoparticles are being used for multiple purposes in the oil and gas industry and may also be included in the cavitation system for improving the efficiency of the process [39]. Nanosized solid particles can selectively adsorb asphaltene and act as catalysts to break the structure of heavy molecules [40,41]. At the nano-scale, exceptional properties can be obtained, such as a high surface area to volume ratio and dispersibility, in addition to high thermal and chemical stability and dispersibility. The reactivity of particles with nanometric dimensions is significantly enhanced because the functional groups on the material’s surface are hindered less as the size of the particle decreases. In other words, atoms on the surface of the nanomaterials are not surrounded by other atoms of the same material and can, therefore, interact with other compounds [42]. Also, due to their small size (1-100 nm), nanoparticles may be employed in both subsurface and surface conditions [43].
In this way, in the specialized literature only Askarian et al. [39] have evaluated nanoparticle usage in a cavitational cracking process. The authors employed iron oxide nanoparticles and used gasoline as a hydrogen donor, finding that the HO viscosity may be reduced by up to 20% and that the presence of nanoparticles lead to an increase in hydrogenation and/or cracking reactions. Nanoparticles of PdO and/or NiO supported on silica [44], alumina and titania [45], have been synthesized for the adsorption and subsequent catalytic decomposition of heavy compounds. Functionalized nanoparticles show enhanced adsorption capacity and high catalytic activity in the reduction of the decomposition temperature of refractory compounds of crude oil such as asphaltenes and resins. Different physicochemical properties of nanoparticles of the same chemical nature play a more critical role in the n-C7 asphaltene adsorption than in catalytic cracking [41].
Nevertheless, functionalized nanoparticles have not been employed for improving the performance of the cavitation process. Therefore, the main objective of this work is to use NiO-functionalized silica nanoparticles for the enhancement of the cavitation process to reduce HO and EHO viscosity. NiO active sites on the surface of the silica nanoparticles lead to augmented adsorption of asphaltenes and contribute to cracking and their conversion into lighter compounds.
2. Methodology
2.2. Materials
NiO nanoparticles were synthesized over a 7 nm silica support (Sigma-Aldrich, St. Louis, MO) using a salt of nickel nitrate (Ni(NO3)2∙6H2O, Merk KGaG, Germany) as has been reported in previous studies [40].
A HO with approximately 40% emulsified water and 13 °API was used to evaluate ultrasound cavitation performance for viscosity reduction with and without nanoparticles in order to use the emulsified water as a hydrogen donor and evaluate droplets coalescence caused by cavitation and nanoparticle usage alongside viscoelastic network disruption as additional mechanisms in viscosity reduction.
The HO selected for the viscosity reduction tests was tested for saturates, aromatics, resins and asphaltene (SARA) content through a micro-deasphalting technique with n-heptane coupled to thin-layer chromatography following the IP 469 standard [46] using a TLC-FID/FPD Iatroscan MK6 (Iatron Labs Inc, Tokyo, Japan). SARA analyses were performed on HO samples before and after treatment. The contents of saturates, aromatics, resins and asphaltene were estimated at 18.23 wt%, 23.04 wt%, 41.71 wt%, and 17.02 wt%, respectively.
2.3. Experimental section
2.3.1. Synthesis of NiO-functionalized nanoparticles
NiO nanoparticles were synthesized in a 1 wt% dosage over 7 nm SiO2 nanoparticles using the incipient wetness technique [47]. First, SiO2 nanoparticles were dried for 2 hours at 120°C. A determined amount of the SiO2 was placed in a thin layer on the bottom of a flat flask into which an aqueous solution of Ni(NO3)2 was dripped slowly to guarantee homogeneous impregnation. Finally, the material was dried at 120°C for 6 hours, and further calcined at 450°C for 6 hours to obtain the metal oxide nanoparticles [40]. The hybrid material in this study is named SiNi1, using the initials of the support substance, the symbol of the cation of the resulting metal oxide, and its weight percentage [40].
2.3.2. Nanoparticles characterization
The hydrodynamic diameter of the nanoparticles was measured using a dynamic light scattering technique (DLS) using a NanoPlus-3 (Micromeritics, USA) and X-ray diffraction (XRD) with an XPert PRO MPD X-ray diffractometer (PANalytical, Almelo, Netherlands). Surface area (SBET) was determined using Brunauer-Emmett-Teller (BET) theory [48] through N2 physisorption at -196°C using a surface area analyzer Gemini VII 2390r (Micromeritics, GA, USA) [49]. Characterization of the functional groups on the nanoparticles’ surface was performed using Fourier transform infrared spectroscopy (FTIR) with an IRAffinity-1 FTIR (Shimadzu, Japan) spectrophotometer [50,51]. Additional information on the characterization of nanoparticles can be found in previous publications [40,52,53].
2.3.3. Emulsified heavy oil characterization
The emulsified heavy oil was characterized using droplet imaging through optic microscopy with a Motic BA310 microscope (Motic, Stockholm, Sweden). This process was carried out on the emulsified oil before and after treatment with and without SiNi1 nanoparticles in order to analyze the effect of cavitation and the addition of nanoparticles in the interfacial tension decrease observed through the droplets coalescence.
2.3.4. Cavitation treatment
An Elmasonic E60H sonicator (Elma Schmidbauer GmbH, Singen, Germany) was employed to provide the ultrasound cavitation treatment at 400 W of power and a frequency of 37 kHz. Sonication was applied, varying the samples’ exposure time at 10, 30 and 90 minutes as well as the dosage of SiNi1 nanoparticles at 500, 1000 and 2000 mg/L. Experiments were conducted at atmospheric pressure and room temperature (25°C). Viscosity reduction caused by the addition of nanoparticles but with zero ultrasound cavitation exposition time was also evaluated. The effect of the nanoparticles supported on SiO2 was also evaluated to find a base line. The sample was evaluated for remaining asphaltene content and better performance through micro-deasphalting with n-heptane.
2.3.5. Viscosity and rheology measurements
Viscosity and rheology measurements were performed in a Kinexus Pro+ rheometer using a GAP of 0.3 mm with serrated plate-plate geometry [13,24]. The viscosity of the samples was measured at a constant shear rate of 10 s-1. The degree of viscosity reduction (DVR) was determined according to Eq. (1) [13,24,54]:
where, 𝜇 𝑟𝑒𝑓 and 𝜇 𝑡𝑟𝑒𝑎𝑡 are the reference viscosity and viscosity after treatment, respectively. The effect of the shear rate of the HO was evaluated before and after cavitation treatment with nanoparticles ranging from 10 to 100 s-1. All experiments were performed triplicate and the uncertainties are presented as error bars.
3. Rheological modeling
In order to carry out a phenomenological explanation of the ultrasound cavitation effect on viscosity reduction alongside additional mechanisms involving viscoelastic network disruption and droplets coalescence, different rheological models, namely those proposed by Cross and Carreau, have been used to describe the rheological behavior of the untreated and treated samples, as in previous studies these have been proven to have a good fit [23]. The equations corresponding to these models are shown below:
Eq. (2) describes the Cross model where, 𝜇 (cP) is viscosity, 𝛼 𝑐 (s) characteristic relaxation time, 𝛾 (s-1) shear rate, 𝑚 constant, 𝜇 ∞, 𝛾 (cP) viscosity at infinite shear rate and 𝜇 0, 𝛾 (cP) viscosity at zero shear rate.
Eq. (3) accounts for the Carreau model, where 𝜆 𝑐 (s) is characteristic relaxation time, and 𝑁 a constant.
4. Results and discussion
4.1. Nanoparticles characterization
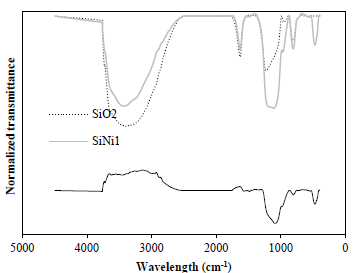
Table 1 shows the properties of the SiO2 and SiNi1 nanoparticles. It is observed that after the functionalization process, the surface area of the nanoparticles is reduced and this could be due to the blockage of some pore space in the support. The mean particle size of NiO nanoparticles over the SiO2 surface was estimated to be 1.9 nm. Fig. 1 shows the FTIR spectrum of SiO2 and SiNi1 nanoparticles, as well as the difference spectrum of both materials. The SiO2 FTIR spectrum shows silica bands assigned to different vibrations in the solid structure. Silanol (Si-OH) groups are observed in the band located at 3780-2470 cm-1 along with an overlapping O-H bond, corresponding to the water molecules [55]. Moreover, a band centered at 1630 cm-1 could correspond to deformation vibrations created by adsorbed water molecules, while the band between 1000 and 1300 cm-1 shows the Si-O covalent bond vibrations. Furthermore, siloxane (O-Si-O) symmetric vibrations appear at about 808 cm-1 [56]. The SiNi1 nanoparticles FTIR spectrum shows significant changes due to the calcination process during SiO2 functionalization. An increase in siloxane groups is observed, this is due to oxidation caused by calcination, as well as a decrease in silanol groups and water adsorbed, generated by the same process [57]. In addition, the increase in the absorption band near 430 cm-1 confirms the presence of NiO nanoparticles in the system as this is related to Ni-O bond vibration [58, 59]. Furthermore, when looking at the difference spectrum, it can be observed that the main bands related to the SiO2 support are subtracted and that bands corresponding to the NiO are exposed.
4.2. Viscosity reduction evaluation
4.2.1. Effect of exposure time
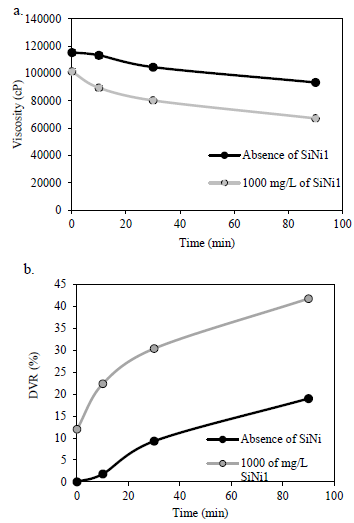
Ultrasound cavitation exposure time was evaluated for 0, 10, 30, and 90 minutes to identify the time range in which DVR is higher. It is worth mentioning that HO without nanoparticles was stirred by hand for 15 minutes, as was the HO with different nanoparticles added. Panels a and b from Fig. 2 show the a) viscosity and b) degree of viscosity reduction for HO before and after the cavitation process with a fixed concentration of 1000 mg/L of SiNi1 nanoparticles for different exposure times. Fig. 2 shows that the viscosity of the sample decreases as the exposure time increases, and this could be due to that fact that as time increases, a higher number of bubbles would be involved in the cavitation process. For the sample that includes SiNi1 nanoparticles, a DVR of 12% is observed when only the nanomaterial is added. This DVR is similar to that of the sample without nanoparticles with an exposure time of between 30 and 90 min. The highest reduction in viscosity (42%) was obtained for the system with nanoparticles and 90 min of exposure time. All experiments were performed in triplicate and the uncertainties are presented as error bars with values < 2%.
4.2.2. Effect of support and nanoparticles dosage
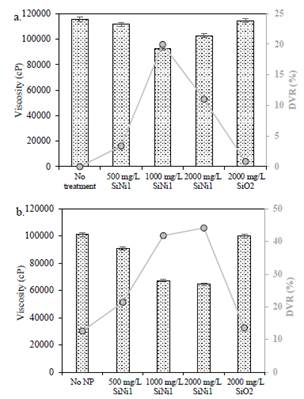
An exposure time of 90 min was selected for evaluating the effect of the SiO2 support and the effect of the nanoparticle concentration. Furthermore, SiNi1 nanoparticle concentrations of 500 and 2000 mg/L were evaluated. Fig. 3 shows the degree of viscosity reduction for a) no ultrasound treatment, b) ultrasound exposure evaluating the effect of nanoparticle dosage and SiO2 support on the HO viscosity reduction. It can be observed in Fig. 3a that a higher viscosity reduction is obtained for the samples that include nanoparticles. These results can be explained due to the adsorption of asphaltenes, a process which disrupts the asphaltene viscoelastic network favoring HO flow capacity [13]. Additionally, it is observed that the higher DVR (20%) was obtained from a 1000 mg/L dosage of SiNi1 nanoparticles. However, when comparing dosages of 1000 and 2000 mg/L in Fig. 3b, the viscosity reduction is 2% higher for the highest dosage, indicating that a concentration of 1000 mg/L could be enough. It is observed that the role of SiO2 role in viscosity reduction is lower than that of the SiNi1 nanoparticles and this is mainly due to the fact that adsorptive capacity and catalytic activity are better in the functionalized material [60].
4.2.3. Effect of droplets coalescence
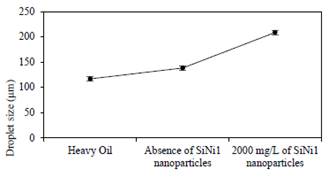
Figure 4 shows the mean droplet size of emulsified heavy oil before and after the cavitation process during 90 minutes with and without 1000 mg/L of SiNi1 nanoparticles. When only the ultrasound cavitation treatment is applied the effect on droplets coalescense is minimal, which minimizes the effect of viscosity reduction from either the demulsifying process or the hydrogenation of free radicals obtained by cracking reactions. However, with the addition of nanoparticles to the system, an appreciable droplets calescence effect was obtained which can be identified by the increase in the size of the droplets, allowing the dissociated hydrogen to freely interact with the HO and futher generating termination reactions with the free radicals obtained from the asphaltenes [36]. Mean droplet size measurements also showed almost negligible errors of less than 5%.
4.2.4. Rheological behavior
The effect of the shear rate on rheological behavior is shown in Fig. 5 for the untreated sample and HO after the cavitation process with 1000 mg/L of SiNi1 nanoparticles. As expected, as shear rate increases, viscosity decreases dramatically, with both fluids exhibiting a pseudoplastic behavior. Additionally, it can be seen that the viscosity of the treated sample is lower than that of the untreated one, corroborating the performance of the catalytic decomposition reaction produced by the ultrasound cavitation treatment alongside the viscoelastic network disruption mechanism.
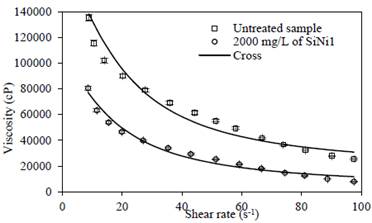
Source: The authors.
Figure 5 Heavy oil before and after the cavitation process with and without 1000 mg/L of SiNi1 nanoparticles. Exposure time = 90 minutes; Temperature = 25 °C.
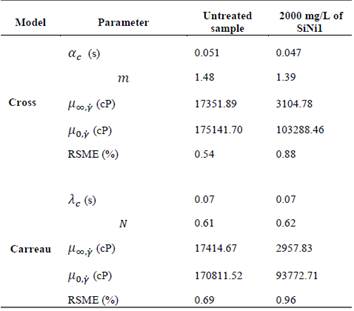
A phenomenological approach to the experimental data was carried out using rheological modeling based on the Cross and Carreau models which in previous studies have proved to be a good fit for the rheological behavior of crude oils.
The rheological model illustrates that the parameters have a logical tendency in which both µꝏ,γ and µ0,γ have lower values than the sample submitted to ultrasound cavitation with 2000 mg/L of SiNi1. Furthermore, a better fit was achieved using the Cross model which can be seen in the lower root-square-mean error values. A decrease in the m constant for the Cross model was observed for the treated sample, which indicates that this fluid showed a more Newtonian behavior, while the αc is similar for both samples, which indicates a similar internal structure recovery time after being subjected to disturbance.
4.2.5. Asphaltene content reduction
The micro deasphalting technique was carried out on the HO before and after treatment to corroborate the catalytic decomposition of the heavy fraction of crude oil. For these experiments, the sample that had obtained better results in viscosity reduction was selected. A decrease in asphaltene content from 17.02 to 14.30 wt% was observed, showing a reduction of 16%. Results indicate that nanoparticles can enhance the decomposition of asphaltenes during the cavitation process. These results coincide with previous studies in which NiO-functionalized nanoparticles have been able to reduce the effective activation energy for asphaltene decomposition, leading to the obtention of lighter compounds and the upgrading of heavy oil [40,44,61,62].
5. Conclusions
This study evaluated heavy oil viscosity reduction through a decrease in its asphaltene content. Its conversion into lighter compounds was achieved through a catalytic decomposition reaction propitiated by an ultrasound cavitation treatment, assisted by NiO nanoparticles. The exposure time, nanoparticle dosage and incidence of emulsified water were studied for their effect on changes in viscosity. Results showed that nanoparticles could reduce viscosity in heavy oils by disrupting the asphaltene viscoelastic network, and by the catalytic decomposition of the heavy fraction after ultrasound cavitation treatment with a dosage of 1000 mg/L. The Cross model described the rheological behavior of the crude oil before and after treatment well. The parameters of the Cross model indicated that after ultrasound cavitation with SiNi1 nanoparticles, the crude oil shows a more Newtonian behavior. Reduction of asphaltene content corroborated that the presence of SiNi1 nanoparticles during the ultrasound cavitation treatment lead to catalytic decomposition of the heavy fraction. The scaling up of this technology can be performed at both surface or sub-surface conditions according to the needs of the oil well and/or oilfield. Further studies should include the effect of other metal oxide and nanoparticle supports, as well as the inclusion of a carrier fluid. This study aims to widen the scope for the application of nanofluids and nanoparticles in the oil and gas industry.