1. Introduction
Removal of organic acids through liquid membranes using tertiary amines has been the focus of several studies [1], especially for the recovery from fermentation systems. Succinic acid has been recovered using a hollow-fiber supported liquid membrane with a trialkylamine [2]. Citric acid has been removed using an emulsion liquid membrane with Alamine 336 [3]. Acetic acid has been separated by emulsion liquid membranes with Amberlite LA-2 (secondary amine), trioctylamine [4], and Aliquat 336 (quaternary ammonium salt) [5]. Lactic acid has been recovered by a supported liquid membrane with trioctylamine [6] and by emulsion liquid membranes with both tributyl phosphate and trioctylamine [7].
In the aforementioned perstraction and reactive liquid-liquid extraction processes, an extractant or carrier is mixed with a diluent [1,4,8,9]. Several characteristics are required from these extractants and solvents, for instance: low viscosity, a high difference of densities with the aqueous phase (that contains the solute), low melting point, medium interfacial tension, high hydrophobicity, thermal stability, low price and availability [10-12], within others. Additionally, toxicity levels of extractant and diluent on microorganisms are key factors to take into account with liquid membranes or liquid-liquid extractions applied for the removal of metabolites from fermentation systems.
Tertiary amines have commonly been used for organic acid removal owing to their high extraction availability, low water solubility and high selectivity [11,13-16] which is usually due to the formation of carboxylic acid-amine complexes [10,17]. Diluents allow for the adjustment of viscosity and density of the solvent phase [15], and on the other hand, provide high distribution coefficients [10]. Diluents can be inert or have an interaction with the extractant that influences its performance. An inert diluent can improve the physical extraction without affecting the transport mechanism [9] while an active diluent can have functional groups that interact with the carboxylic acid-amine complex solvating it in order to stabilize the complex [9,14].
Toxic effects of a solvent on microorganisms are related with accumulation in the cytoplasmic cell membrane. At this level, modifications are made in the functionality of the membrane proteins. Solvents go into and disrupt the lipid bilayer. These compounds generate rupture and metabolite leakage damage. Its effect may result in cell lysis and death [18-20]. The aforementioned toxic effects have been related to a physical property of the substance, the logarithm of the partition coefficient of the solvent in an equimolar mixture of n-octanol and water (Log Pow) [18,21-23]. As lower the Log Pow is, the greater polarity and the toxicity of the solvent will be [22]. The solvent interacts with the microorganism mainly by two routes: direct contact between the cells and the solvent into the aqueous-organic interface (phase toxicity) and by the organic solvent that is soluble in the aqueous broth (molecular toxicity) [19,20,24,25].
When a perstraction process is integrated with a fermentation process (hybrid process), the toxicity of the membrane phase (diluent/extractant) on the microorganisms can rise [10,16]. Thus, additional parameters have to be considered for designing the extraction or perstraction process including loading ratio, complexation equilibrium constant, stoichiometry and rate constant of the organic acid-amine complex [10,26]. In a hybrid process with in-situ product removal (ISPR) [27], either by reactive liquid-liquid extraction or supported liquid membranes, both molecular and phase toxicity affect cell growth and productivity. In order to reduce toxicity, filtration is used for removing biomass from the fermentation broth [25]. However, despite performing the product removal externally to the bioreactor, molecular toxicity can affect the microorganism.
Toxicity of several solvents has been tested on different lactic acid microorganisms. For instance, toxicity of n-dodecane, paraffin oil, chloroform, 1,1,1-trichloroethane, carbon tetra-chloride, ethyl laurate, n-dodecanol, tri-n-dodecylamine, and perfluorodecalin were tested on Lactobacillus delbrueckii ATCC 9649 [28]. Also, the toxicity of Hostarex A327, trihexylphosphate, pentaphosphine-dipentylester, diisotridecylamine, n-octanol, isodecanol, isotridecanol, oleyl alcohol and n-alkanes (C10-C13) were measured on Rhizopus arrhizus CCM 8109 [29]. Toxic effects of Imidazolium-based ionic liquids were tested on Lactobacillus delbruekii NRIC 1683 [24]. Toxicity of Tributylphosphate, tridodecylamine, dioctylamine, tri-n-octylamine, Alamine 336, Aliquat 336, 1-octanol, 1-decanol, 1-dodecanol, oleyl alcohol, n-octane, n-decane, dodecane and kerosene were evaluated on Lactobacillus casei NBMCC-1013 [20]. Almost all these studies (except [28,29]) are based on biomass growth and/or lactic acid production.
Also, it has been shown in the scientific literature that toxicity studies of the several organic solvents on different microorganisms have been contradictory [20], showing that the toxicity is highly related with the type and even the kind of strain of the microorganism. On the other hand, most of the studies have been focused on analyzing the lactic acid production and/or cell growth but rarely glucose consumption has been taken into account.
In this work, the molecular toxicity of three diluents (dodecane, dodecanol, and oleyl alcohol) and three carriers (trioctylamine, tri-iso-octylamine and Aliquat 336), with high potential to be used in lactic acid removal by perstraction or reactive liquid extraction was measured on the bacteria Lactobacillus casei ATCC 393. Also, the molecular toxicity of mixtures trioctylamine/n-dodecane and tri-iso-octylamine/n-dodecane were evaluated at three different volumetric ratios. The toxic effects were analyzed by measuring biomass growth, lactic acid production, and glucose consumption.
2. Materials and methods
Molecular toxicity of n-dodecane (Merck, assay 99 %), 1-dodecanol (Merck, assay 98 %) and oleyl alcohol (Merck, assay 85 %) as diluents and trioctylamine (TOA, Merck, assay 93 %), tri-iso-octylamine (TiOA, Merck, assay 95 %) and Aliquat 336 (Sigma-Aldrich) as extractants were evaluated separately on cell growth of the bacteria Lactobacillus casei ATCC 393 (Microbiologics) using MRS (Sharlau) culture media.
The experimental design for toxicity was first with pure carriers and solvents. For the solvent with the lowest toxicity, the experiments were carried out with amine (TOA, TiOA) concentrations at three levels in the range of concentration reported in the literature for liquid membrane or liquid extraction applications [6,26]. Aliquat 336 was not tested in mixtures with solvent due to its high toxicity. All experiments were carried out with duplicates.
In every single test for each pure substance, 100 mL of culture media was prepared and added into a 250 mL glass flask. Afterward, the organic compounds were added in order to achieve a volume ratio of 1:10 (organic compound:culture media). The six flasks, one for every organic compound (n-dodecane, dodecanol, oleyl alcohol, TOA, TiOA, Aliquat), were shacked using a magnetic stirrer (Velp Scientifica) at 120 rpm and constant temperature of 310 K in an incubator (Binder RI 115, ± 0.3 K) for 72 h (contact stage). Then, the agitation was stopped, each flask was transferred to separation funnels and kept in an oven at 310 K during 72 h for phase splitting (aqueous from the organic phase). Subsequently, the phases were separated.
Volumes of 7.5, 15, 22.5 and 30 mL of each aqueous phase, previously saturated with an organic phase (described above), were taken and mixed with fresh culture media to reach a total volume of 30 ml using Falcon centrifuge tubes of 50 mL. These volumes correspond to proportions of 25 %, 50 %, 75 % and 100 vol%. Then, each culture media was inoculated at 5 vol% with Lactobacillus casei ATCC 393 from an inoculum of 12 h. One control (fermentation without toxic agents) was prepared using fresh culture media. At the beginning of the fermentation (previous to inoculum step, 0 h) and at the end (24 h) one sample was extracted from each Falcon tube (6 (mixtures) x 4 (levels) x 2 (duplicates) = 48 tubes) in order to quantify the glucose consumption, lactic acid and biomass production (in percentage referred to control fermentation). Bacterial growth was measured by optical density (OD at the maximum wavelength, 540 nm) using a spectrophotometer (V1200, Mapada Instruments) and by dry cell weight method [30]. Lactic acid production and glucose consumption were measured by HPLC (ELITE LaChrom) using ORH-801 column (Chrom Tech) with a RI detector at 318.15 K using as a mobile phase a solution of 0.01 N H2SO4 (Merck, assay 95-97 %) prepared with type 1 water (Barnstead™ Nanopure™). Every single Falcon tube was kept in an oven at 310 K for 24 h.
Trioctylamine and tri-iso-octylamine were mixed at concentrations of 10, 20 and 30 vol% with n-dodecane (0 % of amine corresponds to previous experiments with pure dodecane) and its molecular toxicity level were tested following the aforementioned procedure using 30 mL of the aqueous phase (from the phase splitting) for the fermentation step. These concentration of the amine are typical for liquid membranes and liquid extraction [6,13,26]. As it is shown below, Oleyl alcohol and dodecanol were excluded due to their high toxicity compared to dodecane.
3. Results and discussion
Based on the classification of J. Marták et al. [29], organic solvents can be divided into three groups according to their toxicity level on microorganisms. Non-toxic, when the production rate is beyond 75 %, medium toxicity when the production rate is between the 25 % and 75 %, and toxic when the production rate is less than 25 % as compared with a control fermentation.
3.1. Molecular toxicity for pure solvents
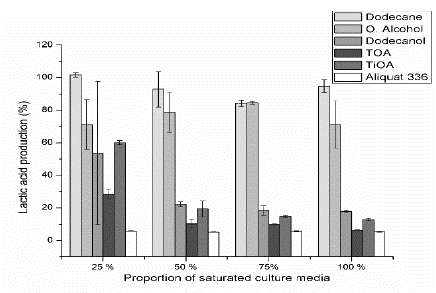
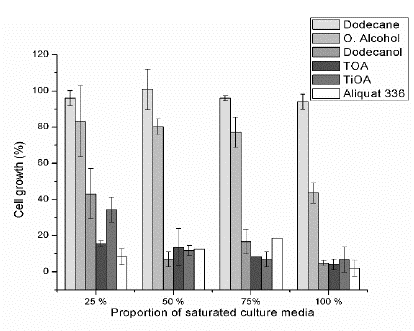
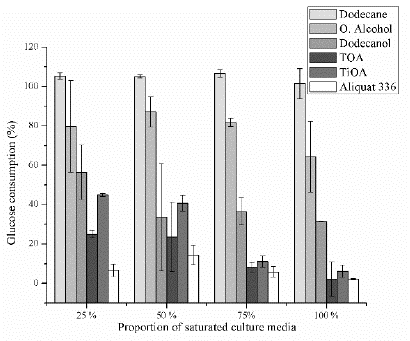
Figs. 1-3 show the relative lactic acid (LA) production, cell growth and glucose consumption of a fermentation broth composed of a mixture of fresh culture media and saturated culture media with the specific solvent at proportions of 25, 50, 75 and 100 vol%, (referred to the saturated culture media). Figs. 1-3 are based on a culture media that were not in contact with the studied organic compounds (control fermentation) and for which the LA production, cell growth, and glucose consumptions were also measured. Taking into account the Marták criteria [29], dodecane was non-toxic for the four tested proportions. Cell growth and LA production were still 16% lower than the control fermentation at the four proportions, while glucose consumption was still 6% higher. For dodecane molecular toxicity test, the glucose concentration in the culture media increases after the contact stage due to the transport of water and some nutrients from the culture media to the organic phase. Thus, the glucose concentration of the saturated culture media was between 1.4% and 5.3 % (SD = 1.5 - 7.6%), higher than the control fermentation, and in consequence, the glucose consumption was promoted achieving values of glucose consumption slightly above the 100 %. Taking into account a higher glucose consumption is expected an increase in biomass and LA production. However, they were till 10% lower but within the standard deviation (maximum SD = 11.2%). The toxicity of dodecane on the bacteria was low enough that it does not have an important effect on the measured fermentation variables.
Source: The authors
Figure 1 Relative lactic acid production for each pure substance at four proportions of the saturated culture media.
Source: The authors
Figure 2 Relative cell growth by dry weight for each pure substance at four proportions of the saturated culture media.
Source: The authors
Figure 3 Relative glucose consumption for each pure substance at four proportions of the saturated culture media.
Oleyl alcohol was non-toxic for volumetric proportions from 25 to 75 % considering LA production, cell growth, and glucose consumption (with a minimum and maximum standard deviation of ±0.9 and ±19.6). However, it can be observed that for cell growth (Fig. 2) at proportions from 25 to 75 %, alcohol can be classified as medium toxic if the standard deviations are taking into account. Oleyl alcohol was medium toxic for a proportion of 100 % based on LA production, cell growth, and glucose consumption (with a minimum and maximum standard deviation of ±5.6 and ±18). The glucose consumption (Fig. 3) was reduced by 20 % for 25, 50 and 75 % proportions and it was reduced by 30 % for a proportion of 100 %. On the other hand, both cell growth and LA production decrease around 20 % for proportions of 25, 50 and 75 % and around 30 % for a proportion of 100 %. These results seem to indicate that the bacteria use glucose equitably to produce LA and biomass when oleyl alcohol is present.
Dodecanol has a medium toxicity for a proportion of 25 %, and it was toxic for the other proportions based on LA production and cell growth (Fig. 1). However, glucose consumption was reduced around 40 % for a proportion of 25 % and around 60 % for proportions of 50, 75 % and 100 %. Also, cell growth rate was reduced by 60 % for a proportion of 25 % and around 90 % for proportions of 50, 75 % and 100 %. LA production was reduced around 50 % for a proportion of 25 % and around 80 % for the other proportions. The aforementioned results show that the bacteria consumes more glucose to survive than the biomass and LA produced. It uses this glucose for maintenance and this behavior is more intense at proportions above 50 % where there are a lower cell growth and LA production as compared to the glucose consumption.
Aliquat 336 (a quaternary ammonium salt mixed with tertiary amines C8-C10-alkyl, octanol, and decanol) was toxic for all tested proportions, taking into account both LA produced and cell growth. LA produced was reduced by 95 % as compared with control fermentation. The consumed glucose was reduced around 95 % for volumetric proportions of 25, 75 and 100 %, but it was reduced by 86 % for a proportion of 50 %. The cell growth was reduced around 95 % for proportions of 25 and 100 % and it was reduced by 88 % for a proportion of 50 %. The aforementioned results show that the bacteria use the consumed glucose to produce biomass but reduce its LA production to survive and for maintenance. For a proportion of 75 % the cell growth was reduced by 82 %. At this point, the bacteria produced an amount of LA in accordance with the consumed glucose but the relative fraction of biomass produced was higher than the consumed glucose. Aliquat 336 was the most toxic amine among tested extractants. Aliquat 336 contains trioctylmethylammonium chloride (TOMAC) that has a chloride ion in its structure and can interact with some substances from the MRS, such as sodium acetate and triammonium citrate to produce sodium chloride and ammonium chloride. Ammonium chloride is soluble in water and increases the medium acidity, whereas low pH can inhibit the lactic acid bacteria [11,16,31].
Trioctylamine (TOA) is toxic for the four volumetric proportions tested. Based on the cell growth, it is medium toxicity for a proportion of 25 % in terms of LA production and it is toxic for the other proportions. However, this value of medium toxicity could be read as toxic considering the standard deviation. The consumed glucose is used to equitably produce LA and biomass for proportions of 75 and 100 %, and as well for a proportion of 50 % if the standard deviations of glucose consumption and cell growth are taking into account. For a proportion of 25 %, the glucose consumption and LA production were reduced by 75 % but cell growth was reduced by 85 %, showing that the bacteria reduces its biomass production for maintenance.
Tri-iso-octylamine (TiOA) was the only extractant that exhibited a medium toxicity. It was medium toxic for a proportion of 25 %, the other proportions and the other amines were toxic based on both, cell growth and LA production. TOA and TiOA are tertiary amines with the same structural formula thus it can be expected the same toxicity level. However, TiOA was less toxic than TOA. The iso structure into the alkyl group for TiOA can produce steric effects that probably reduces its toxic effects.
Although it is known that toxicity of organic solvents depend on the type of microorganism or even of its strain [10,29,34], generally the toxicity is related to the solvent polarity (related to log Pow values - Table 1) [1,19,24], and its water solubility [10,25]. Therefore, it is expected that dodecanol and oleyl alcohol, which are polar, were more toxic than dodecane (non-polar). The toxicity with the alkane is similar to other studies where alkanes were non-toxic or low toxic on bacteria [29,35]. For alcohols, it is known that its toxicity decreases as its long-chain increases [1,29,35,36]. Also, this study shows that oleyl alcohol (C18) is less toxic than dodecanol (C12) on the bacteria Lactobacillus casei ATCC 393 (Fig. 2).
Table 1 Main physical properties of the tested solvents related with its toxicity on microorganisms.
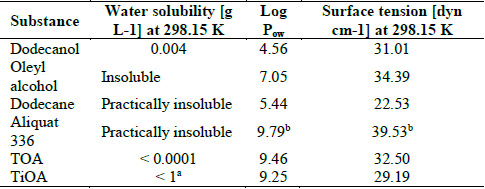
Physical properties were calculated with ProPed-ICAS 14 software (CAPEC-DTU) using Marrero and Gani, Constantinou and Gani methods [32,33]. Water solubility was taken from MSDS. aPhysical property at 293.15 K. bPhysical property calculated for TOMAC using connectivity index neglecting ionic bond.
Source: The authors
The logarithm of Pow (Table 1) is a common parameter to study toxic effects on microorganisms [24,37]. A Log Pow higher than 4, apparently does not have any effect on the biocatalytic activity of the cell [37]. However, a type of Lactobacillus Delbruekii is sensitive to organic solvents with Log Pow between 1 to 4 [24]. Taking into account Log Pow as a parameter of toxicity [24,37], the tested solvents can be considered to be low toxic, except dodecanol that it can be considered as non-toxic due to its Log Pow value, nevertheless, another scientific literature have shown that Log Pow is an unreliable parameter [1] to infer the toxicity level. However, other parameters as solubility and surface tension can affect the microbial growth (biomass and LA production, and glucose consumption).
Surface tension and water solubility have also been used to predict the toxic effect of the solvent on the microorganism. A high surface tension involves a rapid loss of cellular activity [19,37] where the cell membrane fluidity increases [19]. Also, the lower the solvent solubility in water is, the lower the probability of contact between the solvent and the microorganism will be. Thus, microorganisms will be less affected for the solvent presence into the culture media. For these reasons oleyl alcohol is less toxic than dodecanol (water solubility in Table 1) in spite of having a similar surface tension. And dodecane has a low toxicity because it has a lower surface tension and lower water solubility than dodecanol and Oleyl alcohol.
In extractants, the higher the surface tension is the higher the toxicity on the bacteria will be, resulting Aliquat 336 with the highest toxicity in spite of being the extractant with the lowest water solubility. The lower toxicity of TiOA as compared with TOA is due to TOA has a higher surface tension and in consequence, it disturbs the cell membrane function as a barrier, as a matrix for enzymes and as energy transducer [23].
3.2. Molecular toxicity of selected mixtures
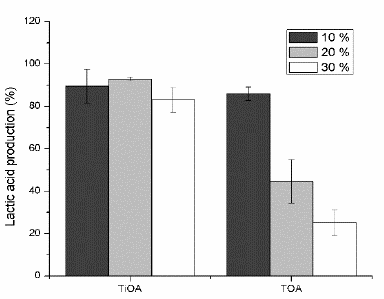
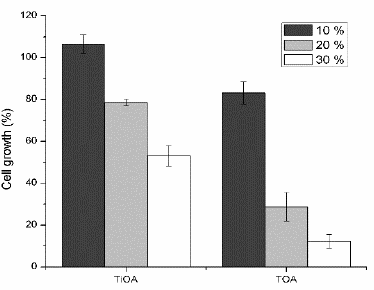
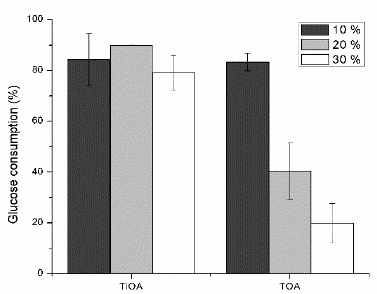
Figs. 4-6 shows lactic acid production, cell growth and glucose consumption (respectively) for the culture media after contact with mixes of TiOA or TOA (less toxic amines) in dodecane (less toxic diluent) at volumetric proportions of 10, 20 and 30 % of the respective tertiary amine in dodecane.
Source: The authors
Figure 4 Lactic acid production for TiOA/dodecane and TOA/dodecane mixtures at three proportions of the tertiary amine.
Source: The authors
Figure 5 Cell growth by dry weight for TiOA/dodecane and TOA/dodecane mixtures at three proportions of the tertiary amine.
Source: The authors
Figure 6 Glucose consumption for TiOA/dodecane and TOA/dodecane mixtures at three proportions of the tertiary amine.
TiOA mixed in n-dodecane was non-toxic at the three volumetric proportions for both, LA production and cell growth. For cell growth, the toxicity effect on the bacteria rises as the TiOA concentration increases, being non-toxic for volumetric proportions of 10 and 20 % and medium toxic at 30 %. There is a LA production according to the glucose consumption for three proportions of TiOA. However, as TiOA concentration rises, the cell growth decreases, perhaps in order to survive due to a high energy consumption of the bacteria trying to tolerate the organic solvent. These microorganisms have developed several mechanisms to increase its tolerance for organics solvents, one of them involves the action of efflux pumps to expel the organic solvent cumulated into the membrane and another one involves to change the membrane rigidity in order to do it less permeable to the organic solvents [21]. Both mechanisms require an additional energy consumption.
TOA was non-toxic at a volumetric proportion of 10 % for LA production and it was medium toxic for both cell growth and glucose consumption. It was medium toxicity at 20 % of TOA and toxic at 30 %. It means that the higher the TOA concentration is the higher the toxicity effect on the bacteria will be. For both proportions 20 and 30 % of TOA, the consumed glucose was used, in higher proportion, to produce LA than biomass. LA production is proportional to the glucose consumed but the biomass production is reduced. This means that for every single cell the LA production is increased as compared to the control fermentation. The metabolic changes inside the cell do not clearly explain this behavior.
4. Conclusions
Taking into account cell growth, lactic acid production and glucose consumption, molecular toxicity level follows this order: dodecanol > oleyl alcohol > dodecane and for the extractants, the order is: Aliquat 336 > TOA > TiOA. Comparing the extractans with the diluents, extractants have higher molecular toxicity level. However, these extractants require to be mixed with a non-toxic diluent that ideally has a small effect on its extraction capacity.
Log Pow is not a convenient property to evaluate the toxicity of the tested solvents. There is not an apparent relation between this parameter and the toxicity of the solvent on the bacteria.
Surface tension and water solubility of the solvents have a considerable influence on the toxicity on the microorganisms. Solvents with a high surface tension or water solubility are more toxic for the bacteria. It means, when a solvent is expected to be toxic for the microorganism, it is convenient to use an organic solvent with a low water solubility.
Tertiary amines, (TiOA and TOA) mixed in dodecane, although were medium toxic and toxic respectively, inhibited cell growth and promoted the LA production (at proportions of 20 and 30 %). Conversely, TiOA at low concentration (10 %) was non-toxic and promoted cell growth instead of LA production.
Both TiOA/dodecane and TOA/dodecane at a proportion of 10 % have potential to be used in perstraction processes due to its low molecular toxicity level, where the lactic acid can be removed externally to the bioreactor from a fermentation broth free of biomass.














