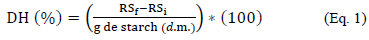1. Introduction
The cassava crop (Manihot esculenta Crantz) has great socioeconomic importance in Colombia due to its outstanding influence on food security policies and income generation in the producing community. In addition, the crop has a high potential at agroindustrial level due to its nutritional contribution and high starch content [1]. Starch is a renewable, biodegradable and economical polysaccharide, biosynthesized in the form of semicrystalline granules composed of two glycosidic macromolecules: amylose and amylopectin [2]. Moreover, it contains small amounts of fiber, protein, lipids, minerals, and phosphates [3-5].
Starch is an important source of energy in human nutrition and is the main storage carbohydrate in cereals (corn, wheat, rice), tubers (potato, tapioca, yam, sweet potato), and also in stems (sago). Native starch extracted from cassava is characterized by its low solubility in cold water, its high tendency to retrograde, low freeze-thawing stability and high viscosity after gelatinization [6-8]. In general, native starches are used to regulate and stabilize food texture due to their thickening and gelling properties. However, they lack well-defined techno-functional characteristics presenting technological limitations. In addition, some dispersions of native starch do not impart an expansion capacity in bakery products where it is sought to take advantage of granule swelling during gelatinization process [1].
Consequently, several methods have been implemented to modify the structure and improve the quality of starches [6,9]. One of them corresponds to enzymatic modification, whose treatments have attracted greater attention due to its safe, ecological and highly controllable nature with the release of harmless byproducts [10]. The biocatalytic action of amylolytic enzymes generates granular disorganization, polymeric degradation and molecular rearrangements of starch granule and influences amylose/amylopectin ratio, crystallinity index, particle size and distribution, morphological changes and increased porosity of the granule [11,12]. In turn, many researchers have evaluated the enzymatic hydrolysis in starches by implementing amyloglucosidase in terms of enzymatic activity, time, pH, temperature and enzyme/substrate ratio [2,13-16].
Amyloglucosidase is an exoenzyme that acts as a biocatalyst in α-D-(1,4) bonds hydrolysis from non-reducing ends of the polymer chain of starch granule. It hydrolyzes α-D-(1,6) bonds that α-amylase cannot attack, excluding the production of limit dextrin [14]. The application of amyloglucosidase in porous starches production has generated promising results increasing water solubility and absorption capacity, providing stability of paste during heating and decreasing its ability to retrograde [8,14,17,18].
On the other hand, the effect of drying type for the extraction and characteristics of hydrolyzed starch has been little reported. However, Gao et al., [18] proved that freeze died porous starch samples showed better properties in terms of adsorption capacity, porosity, and thermal stability. Therefore, they suggest producing porous starches using enzymatic hydrolysis followed by freeze drying. In this context, the aim was to evaluate the combined effect of an enzymatic treatment with amyloglucosidase, followed by a drying process in cassava starch granules on structural, morphological and techno-functional properties.
2. Materials and methods
2.1. Materials
Native cassava starch (Manihot esculenta cv. M-Tai) food-grade was supplied by Almidones de Sucre S.A.S (Induyuca®, Sincelejo, Colombia). Amyloglucosidase food-grade of Aspergillus niger was supplied by Novozymes (Dextrozyme® GA, Bagsværd, Denmark) and potato amylose from Sigma Aldrich (AO512 Sigma, Darmstadt, Germany).
2.2. Characterization of native starch
A bromatological analysis was carried out on samples of native cassava starch that includes the content of ash, moisture, crude fat, crude protein and crude fiber [19]. Amylose content was determined by colorimetric method of iodine at a wavelength of 620 nm [13]. Mineral content was evaluated by atomic absorption spectrophotometry according to the methodology proposed by Singh et al., [20].
2.3. Enzymatic modification
Native starch (10 g) was dissolved in 200 mL of a sodium citrate buffer solution (pH 4.5) at 60 °C with permanent stirring at 250 rpm in a heating-stirring system (Shaker, MaxQ 4450, Germany) for 30 min [14]. Then, 70 uL amyloglucosidase was added keeping the suspension under stirring at 60 °C for 6 or 12 h. Starch suspensions were centrifuged at 7000 rpm for 15 min at 4 °C. Also, solid hydrolyzate was washed twice with distilled water to remove residual enzyme content. Finally, hydrolyzed starch was recovered to evaluate two types of drying. The enzymatic effect is contrasted with control treatments that preserve the operating conditions without the addition of amyloglucosidase.
2.4. Drying processes
Two drying processes were applied to hydrolyzed sstarch granules: (1) Drying by forced convection at 40 °C for 8 h (UFB500, Memmert, Germany), (2) Vacuum drying (VO101, Memmert, Germany) to 50 mBar and 35 °C for 12 h. Drying variables were previously established until a final moisture content of the granule was defined between 11-13% w/w.
2.5. Degree of hydrolysis (DH)
The degree of hydrolysis was determined as a function of the production of reducing sugars by 3,5- dinitro-salicylic method (DNS) [13]. A 500 μL sample reacted with an excess amount of DNS for 8 min at 8 0 °C with subsequent cooling to 10 °C to stop the reaction. Absorbance was determined using a UV-Vis spectrophotometer (UV-2550, Shimadzu, Japan) at 540 nm wavelength. The degree of hydrolysis was expressed as an equivalent percentage of dextrose (DH), where RSi and RSf are the grams of reducing sugars expressed as glucose at the start and at the end of hydrolysis stage (Eq. 1), respectively:
The degree of hydrolysis expressed as yield (Y) was expressed as the ratio between weight at the beginning and at the end of the period of enzymatic action.
2.6. Granule morphology
Samples were analyzed in a scanning electron microscope (SEM) (JEOL, JSU-5600 LV, Japan) according to the method proposed by Chen et al., [2]. Samples were fixed in a sample holder with electro-conductive carbon tape, covered with a platinum/gold alloy. Observation conditions of samples were established at 15 KV, 30 mA and a magnification of 3000X.
2.7. Fourier transforms infrared spectroscopy (FT-IR)
The spectra were obtained in FT-IR spectrometer (Thermo Scientific, Nicolet IS50, US) in the region of 500 to 4000 cm-1, and processed by Thermo Scientific OMNI software [7]. Samples were prepared by a starch/KBr mixture in a ratio of 1:5. 32 readings were made at a resolution of 4 cm-1.
2.8. Swelling Power (SP), Water Absorption Index (WAI) and Water Solubility Index (WSI)
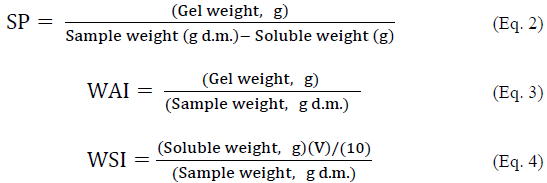
The values of SP, WAI and WSI were determined according to the methodology proposed by Rocha et al., [11] with slight modifications. The starch sample (1 g) was dispersed in 25 mL (V) of distilled water at 60 °C, the suspension was stirred for 30 min, then samples were centrifuged at 5000 rpm for 15 min and pellet was weighed. The SP was estimated as the ratio between mass of wet starch and initial mass of dry starch (Eq. 2). The resulting gel was weighed to estimate WAI (Eq. 3). An aliquot of 10 mL was taken from supernatant, poured into Petri dishes and evaporated in an oven at 70 °C for 16 h. WSI was calculated as the amount of dry solids recovered by evaporating the supernatant from water absorption test (Eq. 4).
2.9. Pasting properties
Pasting properties were determined under the methodology used by Rocha et al., [11] using a micro-viscoamylograph (Brabender GmbH and Co., Germany) with slight modifications. A suspension of starch (4 g bs dissolved in 50 mL of distilled water) was initially undergone a temperature sweep at 50 °C for 1 min, then 95 °C in 7.5 min, maintained at 95 °C for 5.0 min, it was immediately cooled to 50 °C in 7.5 min, and finally kept at 50 °C for 2.0 min. Ascent and descent rate was 7.5 °C/min for each stage. The following parameters were obtained from viscoamylograph: initial pasting temperature (A), peak viscosity (B), final (C), breakdown (D) and setback viscosity (E) viscosities. Experiments were carried out in triplicate and viscosity was recorded in Brabender units (UB).
2.10. Experimental design
A categorical multifactorial design was implemented, and the established factors were: hydrolysis time (6, 12 h) and type of drying (forced convection drying, vacuum drying). Results were analyzed using statistical tools such as Analysis of Variance (ANOVA) and Tukey test for comparison of means with a level of significance of 5% using Statgraphics software version XVI.I.
3. Results and discussion
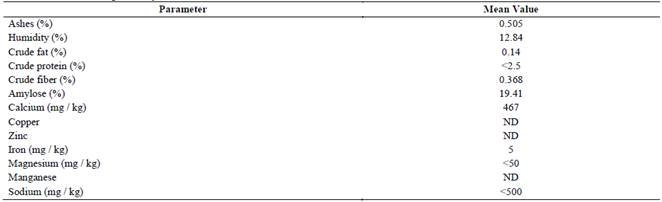
Chemical composition of native cassava starch is detailed in Table 1. A content of moisture, ash, crude protein, crude fat and crude fiber were 12.84, 0.475, 0.11, 0.14 and 0.368%, respectively. This proximal characterization differs slightly from that reported for several cassava cultivars [3,21,22]. These differences can be explained due to several factors that affect the quality of native starch such as variety, age of the crop at harvest time, type of soil, climatic conditions, vegetative health of the tubers or technology implemented in extraction stage [3,6]. However, they are within the permissible limits established in the legislation that regulates the commercialization process of native cassava starch in Colombia [23].
Table 1 Proximate and bromatological analysis in native cassava starch.
Source: Elaborated by the authors.
Minerals such as copper, zinc, and manganese were not identified in the bromatological composition of native cassava starch. Although the presence of minerals such as iron and magnesium at low concentrations (<50 mg/kg) was determined. Additionally, significant concentrations of sodium and calcium were detected between 450 and 460 mg/kg. Previous studies report the identification of minerals such as calcium, sodium, iron, potassium, and phosphorus in starches from different starch sources [4,5]. The presence of minerals such as calcium, sodium or manganese in the chemical composition of starches can be fundamental in enzymatic hydrolysis processes because they increase biocatalytic activity of enzymes such as α-amylase and amyloglucosidase [12,24].
3.1. Effect of enzymatic hydrolysis on native starch
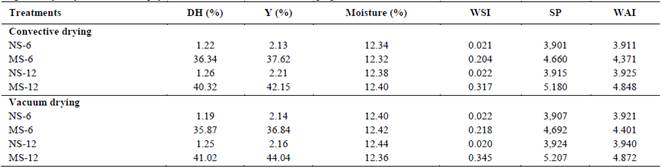
It was determined a significant increase in the production of reducing sugars and in consequence the degree of hydrolysis by catalytic action, on the starch chains. Results consistent with the decrease in yield percentage of modified starch, after enzymatic activity (Table 2). Degree of hydrolysis varied between 35.87 and 41.02% ED for periods between 6 and 12 hours, respectively. Similar values have been reported during the evaluation of enzymatic hydrolysis process of cassava, corn and sweet potato starch granules [11,13,25]. This behavior is probably related to the degradation of amylose and amylopectin fractions by the action of amyloglucosidase, significantly affecting structural and morphological characteristics of starch granules [25].
Table 2 Degree of hydrolysis, behavior of physicochemical and techno-functional properties in native and modified cassava starches.
NS: Native cassava starch, MS: Enzymatically modified starch.
Source: Elaborated by the authors.
Hydrolysis time significantly affected the amylose content in modified cassava starches (p <0.05). These results agree with those reported in cassava starch granules modified by enzymatic way, whose authors suggested that amylolysis occurs mainly in amorphous regions, although part of the crystalline regions also degrades [10]. On the other hand, Shariffa et al., [13] concluded that depolymerization process involves the breaking of glycosidic bonds and intermolecular forces, which results in loss of intragranular order with significant changes in absorption, retention and solubility properties of starchy material.
3.2. Granule morphology (SEM)
Native cassava starch granules have a spherical or oval morphology characterized by a relatively smooth surface (Figs. 1a, 1c). Some starch granules reveal minor damage to their surface with truncated ends, associated with nature or extraction process. These microscopic appearances are similar to those described by Chen et al., [2] and Zhu [6].
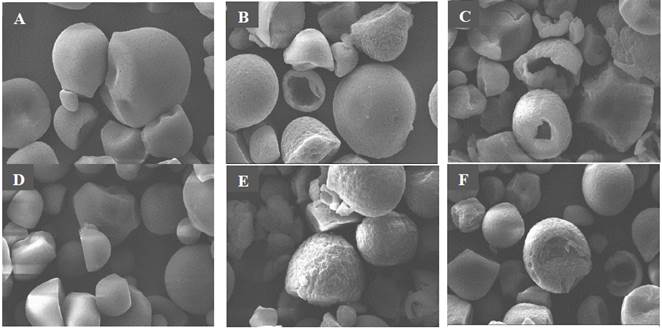
Source: Elaborated by the authors.
Figure 1 Microphotographs by Scanning Electron Microscopy (x3000): A) NS by convective drying, B) MS for 6 h by convective drying, C) MS for 12 h drying by convective drying, D) NS vacuum drying E) MS for 6 h vacuum drying, F) MS for 12 h vacuum drying.
The effect of enzymatic treatment is clearly evident in the microstructure of hydrolyzed granules as a function of hydrolysis time. In Figs. 1b and 1e the significant attack of fungal amyloglucosidase is observed due to the appearance of eroded zones on the external surface of the granule. However, more evident changes are showed with the increase of hydrolysis time, observing small cracks, a greater porosity or partial loss of morphological characteristics of the native granule (Fig. 1c, 1f). Similarly, significant morphological changes have been reported with more porous and deeper structures in granules of modified corn starch with amyloglucosidase [8,17,18]. In other studies, similar results have been reported, where modified cassava granules had openings in the interior that extended to the central cavity with the consequent alteration of morphological characteristics. Furthermore, no significant changes were detected in the external appearance of starch granules treated with buffer solution at pH 4.5, preserving its morphology and surface-level characteristics (microphotographs not shown). Similar results have been reported in corn, cassava, wheat and rice starches treated with sodium acetate buffer for 2 h [8,14].
Drying method seems to slightly affect the microstructural characteristics of cassava modified starches. Photomicrographs of modified starch and dried by air forced convection show the presence of pores and cracks, although porosity is relatively shallow (Figs. 1b, 1c). Whereas porosity and erosion increased in the surface of vacuum-dried modified starches, the size and depth of pores were similar to those observed in air convection-dried starch (Fig. 1e, 1f). Gao et al., [18] report similar results in obtaining porous maize starches by evaluating different drying methods: convection, vacuum, and spray. The same authors argued that vacuum-dried modified granules offered a better water absorption and solubility capacity, because a higher porosity increases the contact surface area, in comparison with native and modified convection dried granules.
3.3. Analysis of spectra by FT-IR
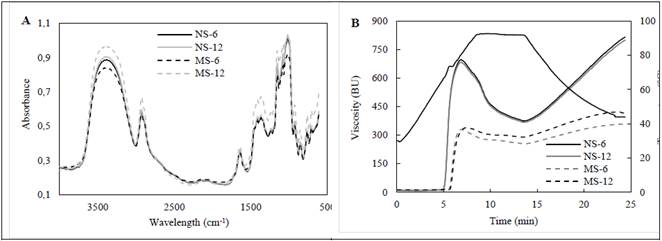
The FT-IR spectra indicates changes in bands and absorption peaks characteristic of starchy materials after enzymatic hydrolysis, possibly associated with molecular and structural changes in starch granule (Fig. 2a, 2b). In absorption bands at 3700 to 3100 cm-1 characteristics of OH bond present in glucose units, a slight narrowing and a marked flattening of the absorption peak are observed in hydrolyzed starches spectra, probably attributed to changes in molecular order of the granule [9,16]. Wavelength range from 2940 to 2910 cm-1 related to vibration of CH bonds diminished their intensity possibly associated to changes in the structural conformation and semi-crystalline order of modified granules [26].

Source: Elaborated by the authors.
Figure 2 Infrared spectra FT-IR in native and modified cassava starches: A) Convective drying, B) Vacuum drying.
Two characteristic peaks of native starch are found in band length at 1646 cm-1 and 1460 cm-1 associated with the presence of water strongly linked to the starch structure [16]. The absorption peaks in modified starches decreased their intensity compared to control sample due to the possible incorporation or substitution of OH groups that influenced water retention and absorption properties [26]. Peaks located at 1010 and 1160 cm-1 are attributed to vibrations of CO, CC or OH functional groups, whose chemical bonds are present in amylose and amylopectin molecules [9,27]. Mu et al., [26] argue that in enzymatically modified starches these bands suffer tension or bending vibrations due to the breaking of α-D-(1,4) glycosidic bonds that form polymeric chains product of the biocatalytic action of some hydrolases such as α-amylase or amyloglucosidase.
Source: Elaborated by the authors.
Figure 3 Pasting properties in native and modified cassava starches: A) Convective drying, B) Vacuum drying.
Absorbances in the region of 1047/1022 cm-1 allow to estimate the ordering degree of the granule, where band 1047 cm-1 is associated with crystal structure, while band 1022 cm-1 is associated to the sensitive amorphous structure [27,28]. According to the techniques established by Sevenou et al., [27] and Sun et al., [29], crystallinity index (CI) was estimated from FT-IR spectra. An increase in CI was determined after enzymatic treatment (Table 3). Furthermore, spectra show displacement of bands in this region by link vibrations -COC- present in polysaccharide materials formed of D-glucose units. These results allow to infer possible conformational changes in the molecular order resulting from the breakdown of glycosidic bonds during the conversion to reducing sugars, specifically affecting semicrystalline order of granules of modified starches. Similar results have been reported in potato hydrolyzed starches, due to structural changes associated with depolymerization of the amorphous regions of granule during the enzymatic attack [9].
Table 3 Pasting properties in native and modified cassava starches.
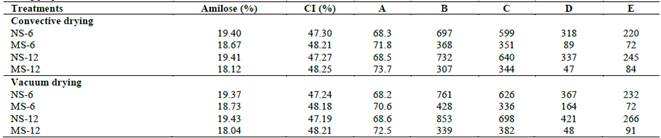
Note: IC (%) estimated by FT-IR spectroscopy. Initial pasting temperature (A), peak viscosity (B), viscosity at 95 °C, 13.5 min (C), breakdown viscosity (D) and setback viscosity (E).
Source: Elaborated by the authors.
Bands or absorption peaks characteristic of starchy materials did not show any changes independent of the drying method applied after enzymatic action (Fig. 2a, 2b). The FT-IR spectra allow us to infer that the drying conditions studied did not cause significant changes in the molecular order of starch granules. Therefore, there is no differences in structure and semicrystalline profile between vacuum-dried and air convective-dried modified starches.
3.4. Swelling Power (SP), Water Absorption Index (WAI) and Water Solubility Index (WSI)
The swelling power increased significantly with degree of hydrolysis in modified starches with respect to control (Table 2). Similar results have been reported after enzymatic modification of cassava starch granules, corn and sago [10,14]. It has been confirmed that the swelling capacity of starch granules is a function of amylose content, amylose/amylopectin packaging and molecular structure of amylopectin [20,30]. Results indicate that enzymatic hydrolysis can alter both the crystalline and amorphous structure of starch granules, affecting their ability to capture water and swell during heating [31]. In turn, Tester and Morrison [30] found that a high amylose content can inhibit swelling power. Consequently, changes in SP treated samples are probably linked to a higher or lower amylose content, relative to control samples.
Biocatalytic activity of amyloglucosidase significantly affected hydrophilic properties of cassava starch, increasing its ability to bind or absorb water molecules. Wang et al., [32], Jung et al., [15] and Benavent and Rosell [8] state that the properties of water solubility and absorption in starches treated with α-amylase or amyloglucosidase are linked to greater porosity or lacerations caused on the surface of granules that influence contact surface area. Furthermore, solubility represents the proportion of polymeric materials leached during swelling with the ability to solubilize in water [1]. This indicates that the enzyme attacked glycosidic bonds breaking intermolecular bonds, facilitating the release or availability of soluble polymer components of starch [9,15].
No significant differences were detected with respect to the drying method applied in the behavior of techno-functional properties related to starches hydrophilic capacity (Table 2). The behavior of WSI can provide information on the degree of interaction of polymer chains in amorphous and crystalline regions of starch granules [6,30]. Likewise, starches that have a high swelling power and a good water absorption index affect pasting properties, as indicated by the results for hydrolyzed starches.
3.5. Pasting properties
Viscoamylographs of native and modified cassava starches show the characteristic behavior of viscosity with temperature of the starch/water suspension commonly observed during gelatinization step (Fig. 3a, 3b). A significant increase in the initial pasting temperature was observed in hydrolyzed starches with respect to control, possibly due to structural changes of amorphous and crystalline zones as shown in FT-IR spectra (p <0.05). Prado et al., [33] argue that an increase in crystallinity leads to greater packing, forming a denser molecular domain that prevents heat transfer, causing an increase in gelatinization temperature. In turn, enzymatic processes mainly alter the order of double helices present in amorphous zones, disturbing the diffusion process of water molecules, as well as the conditions of endothermic process during starch granule gelatinization [2,18].
Maximum viscosity decreased significantly after the enzymatic attack (p <0.05). Similar results were reported in porous corn starches, associated with changes in the surface of granules that increase water retention capacity. This causes amylose and amylopectin to solubilize in the aqueous phase, affecting SP and the adhesion of granules for the formation of network structure at the beginning of gelatinization process [8,14]. This behavior in hydrolyzed starches may also be related to the degradation of amorphous areas, as suggested by the analysis of amylose content and FT-IR spectra. Depolymerization of amylose and amylopectin in shorter chain lengths causes a weakening at molecular level reflected in a lower viscosity of suspension granules [10,33]. In addition, a displacement of the maximum viscosity peak for modified starches was observed, related to the need to increase heating period and gelatinization temperature.
In the viscosity profile of modified starches, no abrupt drop in viscosity was observed at the end of the constant heating period, with respect to peak viscosity (breakdown viscosity). This indicates that starch granules have good structural stability and are less susceptible to rupture or breaking at shear forces during continuous heating [10,11]. This behavior is related to the biocatalytic effect on amorphous structure, increasing molecular order and mechanical resistance of granules subjected to thermal and mechanical stress. Retrogradation in hydrolyzed starches decreased significantly, analogous behavior with the possible degradation of amylose during hydrolysis. Retrogradation is a process that partially depends on amylose content, length and dispersion state of the linear chains that make it up [6,7]. Similar results have been reported in porous and hydrolyzed starches from diverse starch sources, suggesting a drop in setback viscosity due to the weak rearrangement or crosslinking of bonds in linear amylose chains solubilized during heating [8,11].
Drying process slightly affected the pasting properties in native and modified cassava starches. A similar behavior was analyzed in obtaining porous corn starches, citing that vacuum-dried granules or freeze drying showed a better stability in terms of viscosity with temperature compared to samples dried by air convection [18]. Researchers explain that these differences are possibly associated with changes in water absorption and solubility due to increased porosity or lacerations (exo-corrosion) on the surfaces of starch granules.
4. Conclusions
The enzymatic activity time had a significant effect on the degree of hydrolysis in granules of native cassava starch, reporting values of dextrose equivalents between 35 and 41% treated below gelatinization temperature. Enzymatic treatment during 12 h was the most severe, causing a disorganization in the polymeric and semicrystalline structure of the granule, and affecting the morphology of native granule. Likewise, biocatalytic process improved properties as SP, WAI, and WSI in hydrolyzed starches causing significant changes on the initial gelatinization temperature, maximum viscosity, and breakdown viscosity. In general, morphological properties were not affected by the drying method, although a more homogeneous porosity was observed in vacuum-dried starch granules at surface level. Results show that hydrolyzed starches followed by a vacuum drying process presented a better hydrophilic capacity in terms of solubility, affecting peak viscosity and ability to retrograde on starch granules.