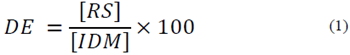1. Introduction
Bioethanol production by the transformation of biological resources such as corn, sugarcane, and sugar beet or sorghum or via hydrolytic pretreatment of lignocellulosic biomass as second-generation source (2G) followed by enzymatic conversion [1] is a promising alternative for fossil fuels [2]. In this sense, feedstock such as barley, wheat, rice, and tuber crops (i.e., potato and sweet potato) may serve as better options since they are more abundant and can be acquired at a lower cost [3-6].
The potato is a potential feedstock for ethanol production due to its high starch content (approximately 80%) and a yield that is two to three times higher than that of fermentable sugars such as field corn [7,8]. Moreover, potatoes have many agronomic features, including high multiplication rate, drought resistance, and low degeneration rates of the planting material [9,10]
Because starch is an important substrate for the fermentation process, researchers have investigated a two-stage process for potato starch saccharification with acid pretreatment and enzymatic hydrolysis for ethanol production [11-13] and for the production of hydrolysates such as maltose and glucose [14-21], whose distribution depends on the acidic/enzymatic conditions of the process. These previous processes produced a solid insoluble fraction of cellulose and fibers and a liquid fraction composed of soluble sugars (mainly glucose), which is used in submerged fermentation to produce metabolites.
Although the process has been well defined, the conversion of insoluble starch granules into polymer fragments and its subsequent breaking into reducing sugars (saccharification) suffers from certain technological inconveniencies, such as successive pH and temperature changes to maximize the hydrolytic enzymatic system, which results in an increased consumption of energy and auxiliary materials used in the purification of hydrolysates [14]. However, an option for overcoming these difficulties could be the use of a single-stage method for starch hydrolysis using a simultaneous saccharification-fermentation (SSF) process. Another important limitation during the process, is the generation of undesirable branch points during liquefaction, such as α-1,6-glycosidic links (4-6%), which must also be cleaved to complete hydrolysis; however, they are not attacked by the enzymatic system, because most hydrolytic enzymes are specific for α-1,4-glycosidic links [22-24].
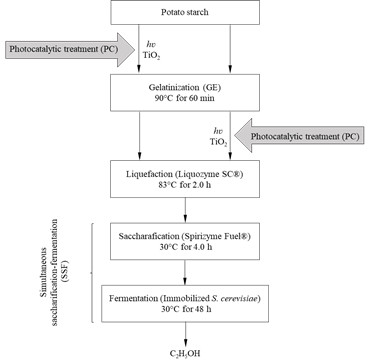
Whereas photocatalytic (PC) pretreatment has been used to transform organic waste [25-28] and complex structures such as lignocellulose [29], its application for modifying starchy materials used for bioethanol production by OH• radicals participation, has rarely been reported. In this work, the effect of PC treatment, with TiO2 before and after the gelatinization (GE) process during SSF, on bioethanol production from potato starch was tested (Fig. 1).
2. Materials and methods
A commercial sample of industrial potato starch (Almicor, Bogotá, Colombia) with a water content of 8.3% and 97.0% starch was used. HCl (Carlo Erba, Italy) and NaOH (Merck, Germany) solutions were used for pH adjustment during each stage.
For photocatalytic pretreatment, TiO2 (Degussa-P25) was used. The commercial enzymes for liquefaction (Liquozyme SC, 167 kilo Novo α-amylase unit KNU ml-1) and for saccharification (Spirizyme Fuel, 953 Novo glucoamylase unit AGU ml-1) were purchased from Novozymes, USA [30]. Immobilized yeast cells of dry S. cerevisiae (Fermentis, Ethanol Red, France) in Ca-alginate gel beads were employed for reducing sugar fermentation. Typically, a 4% sodium alginate sterile solution (weight fraction) was mixed with an S. cerevisiae (YSC1, Sigma) suspension (30 mg dry biomass ml-1 alginate solution) and extruded through a needle (21 G) into a flask containing 0.1 M CaCl2 sterile solution at 25°C to form microspheres, which were moderately shaken for 30 min [31]. (NH4)2HPO4, MgSO4·7H2O and KH2PO4 (Merck, analytical grade, Germany) were used as nutrients during the fermentation process.
2.1 Photocatalytic (PC) pretreatment experiments
PC treatment was applied before and after the gelatinization stage (GE) using TiO2 Degussa P-25 with photocatalytic activity. For the PC→GE pretreatment, 200 ml of potato starch-water suspension (~17%) was mixed with TiO2 for 15 min to obtain a homogeneous paste (0.1 g TiO2 g starch-1). A sample, was spread in a thin layer on a glass plate, covered by another glass plate and subsequently irradiated for 5.0 h in a solid state from above the plates by a black-light blue fluorescent lamp (λ=360 nm, Phillips, Germany). Then, the sample was isothermally incubated at 90°C with mechanical shaking at 350 rpm for 60 min (GE). For the GE→PC pretreatment, 200 ml of potato starch-water suspension with the same characteristics was subjected to GE (90°C, 350 rpm, 60 min). After the incubation, the suspension was mixed with TiO2 to obtain a paste (0.1 g TiO2 g-1 gelatinized starch), which was irradiated under the same conditions described above.
2.2. Liquefaction
All of the resulting mash after PC pretreatment, without separation of TiO2 [29], was stabilized at 60°C and pH 5.8 [32] and mixed with 10.0 ml l-1 Liquozyme SC (5.6 KNU g l-1). The mixture, containing approximately 51.0 g l-1 total solids, was liquefied at 83°C for 2.0 h. The enzyme activity was inactivated by adjusting the pH (~4.3) with 1.0 M HCl. Aliquots of the supernatant were separated by centrifugation (8000 rpm for 5 min) and used to determine reducing sugar content using a DNS method, relative to a glucose standard curve. The dextrose equivalent (DE) value for the treatments was calculated as the amount of reducing sugars (g) expressed as a percentage of the initial dry matter (g) according to the following equation (eq. 1):
where RS and IDM are the reducing sugar and initial dry matter concentrations, respectively. Prior to the SSF process, the liquefied mash was cooled at room temperature for 1.0 h. Deionized water was added to adjust the total solids to approximately 0.4 g l-1. The final pH of the liquefied mash was 4.5-4.7, and no further pH adjustment was made.
2.3. Simultaneous saccharification and fermentation (SSF)
Batch scale ethanol fermentation of the liquefied mash was performed under SSF conditions after the liquefaction step.
Source: The authors
Figure 1 Enzymatic processes for bioethanol production from potato starch involving PC pretreatment before (PC→GE) and after (GE→PC) the gelatinization stage.
The liquefied suspension was dispensed into an Erlenmeyer flask with a rubber stopper [33-36]. Saccharification was then initiated by adding Spirizyme Fuel (1.5 ml l-1, 2.27 AGU g-1 available starch), 6.0 g l-1 (NH4)2HPO4, 2.0 g l-1 MgSO4∙7 H2O, 3.0 g l-1 KH2PO4 and an inoculum of yeast immobilized microspheres (8.0 g l-1) [37]. The final total solids content of the mixture was approximately 0.42-0.45 g l-1, providing an available glucose concentration of 0.36-0.38 g l-1. SSF was performed over 48 h at 30°C, with an initial pH of 4.5-4.7 and a shaker speed of 150 rpm. The ethanol concentration was monitored at 6.0-h intervals. The kinetic parameters for the bioreactor design, such as maximum specific growth rate (ʋmax) and the Michaelis-Menten constant (Km), were estimated based on a mathematical model that describes the estimation of substrate conversion per unit time in a batch reactor [38-41]. A reference experiment was carried out without PC pretreatment, to compare the effect of TiO2 on SSF of potato starch for bioethanol production. All experiments were conducted in triplicate.
2.4. Analytical methods
The fermentable sugar content was determined by acid hydrolysis in which the samples were treated with HCl at 100°C for 2 h and the amount of reducing sugar was measured by the DNS method (3,5-dinitro salicylic acid) using glucose as the standard [42] in a Lambda 750 UV/Vis/NIR Spectrophotometer (Perkin Elmer, USA). All measurements were recorded at 540 nm. Fermentation samples were taken from the bioreactor and centrifuged at 7000 rpm to remove any solids from the media. All determinations were performed using standard curves [43]. A sample of the supernatant (0.8 ml) was filtered through a 0.45-mm membrane filter (Millipore, USA) and mixed with 0.2 ml of n-propanol. A gas chromatograph (model Clarus 580 Gas Chromatograph (GC, Perkin Elmer, USA), equipped with an Elite-Wax ETR column (60 m, 0.25 mm ID, Perkin Elmer, USA) connected to a flame ionization detector (FID) was used to determine ethanol concentration. The detector and injector temperatures were adjusted to 200°C. The detection limit of the method was determined to be 40 ppm.
3. Results and discussion
3.1. Effect of photocatalytic pretreatment on SSF of potato starch
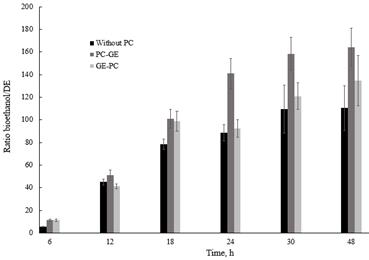
Considering that starch content was the same for all treatments, a productivity ratio was determined based on the bioethanol concentration and dextrose equivalent (DE) during the process (Fig. 2). The results showed that bioethanol productivity varied significantly with photocatalytic pretreatment. Ethanol productivity during the process at 48 h was 164.2, 134.6 and 110.4 gbioethanol (greducing sugars gstarch)-1 for PC→GE, GE→PC and without PC pretreatment, respectively.
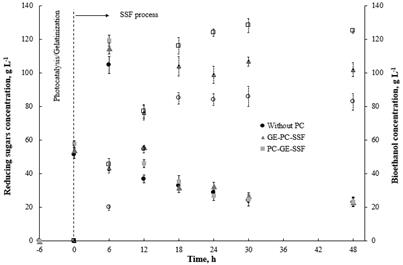
The levels of the reducing sugars were detectable after the gelatinization stage and during the SSF process. They accumulated progressively but decreased after 6.0 h until 30 h. At this point, they reached a basal level (~20 g l-1) that was maintained over the next 48 h. The maximum amount of reducing sugars was 119.25, 114.62 and 104.8 g l-1 for PC→GE, GE→PC and the reference (without PC), respectively (Fig. 3).
The bioethanol was detectable after 6.0 h of fermentation, and higher concentration levels of bioethanol were achieved when PC pretreatment was applied. These levels increased gradually up to a maximum amount of 128.21, 106.74 and 85.91 g l-1 after 30 h, for PC→GE, GE→PC and the reference (without PC), respectively. Consistently, with the reducing sugars concentration present in the media, the bioethanol concentration increased slightly during 18-30 h of fermentation, suggesting that all-reducing sugars were converted to bioethanol or that undesired products, such as ethanol, inhibited the enzymatic action.
Source: The authors
Figure 3 Effect of photocatalytic pretreatment with TiO2 on potato starch and ethanol production. Each experiment was performed three times.
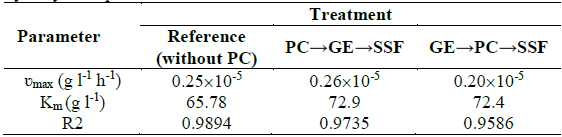
Table 1 ʋmax and Km values for the estimated kinetic parameters for the enzymatic hydrolysis of potato starch.
Source: The authors
This result indicated that bioethanol production was greater when the photocatalytic pretreatment was applied. Compared with the reference treatment and other studies on potato starch fermentation [7,44], we found that ethanol productivities and yields could be influenced by hydroxyl radicals generated during photocatalytic pretreatment, which could transform important structures during irradiation and accelerate the breakdown of α-1,6-glycosidic linkages, making them more susceptible to enzymatic attack during saccharification [45].
Although reducing sugars and the bioethanol concentration obtained from potato starch were similar to those attained from traditional enzymatic fermentation [9], the effect of photocatalytic pretreatment could be related to the shortening of SSF time, which was established at approximately 30 h of treatment.
3.2. Estimated kinetic parameters for bioethanol production from potato starch
The experimental concentration of reducing sugar was used for graphical correlation of the kinetic constants according to linearization of the Michaelis-Menten model, where the maximum rate or velocity of reaction of the enzymes and the Michaelis-Menten constant are ʋmax and Km, respectively. Table 1 shows the values obtained for each treatment according to data fitting.
The maximum rate reaction of the enzymes (ʋmax) was similar for all treatments. According to the Michaelis- Menten constant (Km), the enzyme showed less affinity with the available substrate in PC pretreatments than with the reference (without PC). However, the low conversion of reducing sugars with the reference and, in contrast, the higher bioethanol concentration in PC pretreatments, suggested that TiO2 may have altered the biological reactions of the yeast, which implies that the production of ethanol could be further improved.
4. Conclusions
Based on the productivity ratio, the bioethanol production from potato starch was improved by PC pretreatment with TiO2 before the liquefaction process. The values obtained for the kinetic parameters regarding batch conversion of potato starch into ethanol showed that the enzyme activity (ʋmax) reaction rate without pretreatment does not promote quick substrate conversion into ethanol. In contrast, although the reaction rate was slow for PC pretreatments, it reflected the highest substrate conversion into product. Considering traditional potato starch hydrolysis, PC pretreatment shortened the reaction time of the biological reactions. Thus, the PC pretreatment of potato starch for bioethanol production could be an environmentally aware process without acid and alkali.