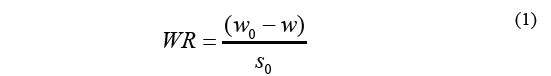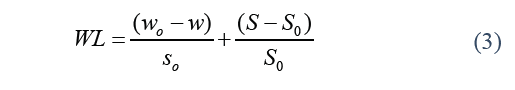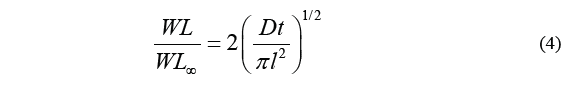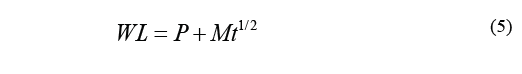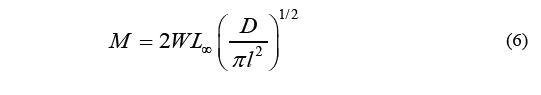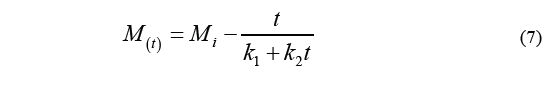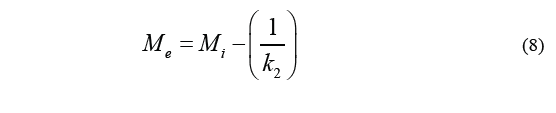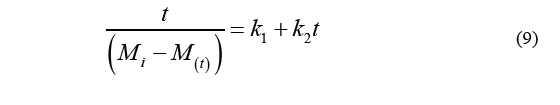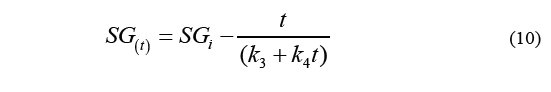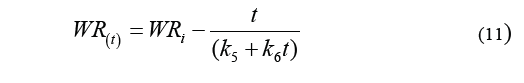1. Introduction
Auyama (also called Caribbean pumpkin) offers numerous qualities for the agri-food industry, the health care industry and agribusiness [1]. This vegetable is native to the Americas, and comes in numerous varieties that can be found throughout the continent. This low-calorie vegetable is composed mainly of water, carbohydrates, proteins, fiber and negligible fat. Auyama is considered to be a good source of fiber, which improves digestive health, as well as a good source of Beta-carotene or pro-vitamin A (essential for good vision and healthy skin), ascorbic acid, and vitamin E (which has antioxidant properties and supports a healthy immune system). It also has a wide range of microelements such as phosphorus, iron and calcium, which are essential for the formation and maintenance of bones, hemoglobin and skeletal system, respectively [2]. However, despite all its properties, this vegetable has a considerably high moisture content (approximately 90%), which makes it a perishable food and limits its use in industrial processes in addition to having a reduced lifespan [3].
Osmotic dehydration is a process that can help reduce the high moisture content of these vegetables and help solve problems related to technological development, as it is aimed at reducing water activity of foods as well as the rates of chemical reactions, therefore facilitating storage and distribution [4,5]. On the other hand, it could be said that mass transfer occurring in osmotic dehydration is triggered by the driving forces associated with the difference in the chemical potential of foods and the hypertonic solution (higher concentration of solutes) [6]. Therefore, water and solute activity gradients are responsible for causing the osmotic flow of water that occurs in the cell membrane.
Additionally, osmotic dehydration is used as a treatment prior to drying processes in fruits and vegetables, which are common in the food industry, including hot air drying, microwave drying and lyophilization, among others. This pretreatment is carried out prior to drying processes in order to help preserve and improve sensory and nutritional properties of an end product. This reduces the damages caused by heat, including changes in color and taste of foods, which can significantly affect product quality [7,8]. It is worth mentioning that osmotic dehydration consists of immersing a solid food in a hypertonic solution over a specific period of time in order to help remove water. Simultaneously with water expulsion, solid particles penetrate the food due to the osmotic pressure, resulting in an intermediate moisture product [9,10]. Therefore, the solute forming the hypertonic solution must be chosen carefully paying special attention to the impact sought with respect to the sensorial characteristics of the end product and the costs associated with the use of the selected solute. Considered the above mentioned, the aim of this project was to address issues such as the modeling of weight reduction (WR), solid gain (SG) and weight loss (WL) in osmotic dehydration of auyama by ternary solutions.
2. Materials and methods
2.1. Sample preparation
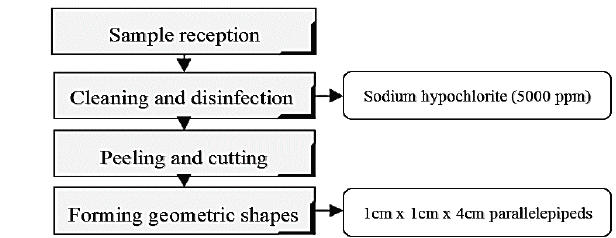
The experiment involved the use of samples of auyama from local grocery stores. Samples were classified according to their shape and size by selecting those with healthy peels. Fig. 1 describes the procedures to which raw materials were subjected in the lab.
The selected samples were placed in a desiccator in order to perform the subsequent osmotic procedures.
2.2. Osmotic dehydration process
Hypertonic solutions were prepared prior to dehydration process. The first solution consisted of a mixture of stevia, salt and water (S1) while the second solution consisted of glucose, salt, and water (S2), at the following concentrations: 20% w/w (15% sweetener - 5% salt), 30% w/w (25% sweetener- 5% salt) and 40% w/w (35% sweetener - 5% salt). The sample-solution ratio was 1:20 w/w, 40-160-min processing times, 40-min intervals. Constant stirring speed at 100 rpm was used. Subsequently the samples were removed and drained on absorbent paper for 1 min, and then packed and deposited into a desiccator. Then, weighting and moisture analysis were carried out according to Colombian Institute for Technical Standards and Certification (ICONTEC) standard NTC 572.
2.3. Calculating kinetics
The Eq. (1) was used to calculate osmotic dehydration kinetics and determine weight reduction.
Where WR stands for weight reduction, w o is the initial matter weight (g), w is the weight of the material over a specific period of time t (g), and S o stands for the initial weight of the dry sample (g). Solid gain was calculated using the Eq. (2).
Where SG stands for solid gain, S is the weight of the dry sample over a specific period of time t (g), and S o stands for the initial weight of the dry sample (g). Water loss was calculated by the Eq. (3).
Charts for kinetics corresponding to WR and SG were also made.
2.4. Determining diffusion coefficient
Diffusion coefficient for each treatment was calculated by the method proposed by Maldonado [11], which uses the standard Fick model to obtain the diffusion coefficient based on the square root of time. Therefore, the diffusion coefficient was determined by calculating the slope of the resulting lines, as described in the Eq. (4).
Where D is the diffusion coefficient, t stands for time (min), WL ∞ stands for water loss at the time of reaching equilibrium, and WL is the loss of water over a specific period of time t. Therefore, the above equation can be transcribed as Eq. (5).
Where P is the intercept with the axis of the ordinates, and M is determined as Eq. (6).
Kinetics approaching asymptotic equilibrium was calculated by Peleg's equation [12], which proposes a two-parameter non-exponential model as described by the Eq. (7) [13].
Where M i stands for the initial moisture content, M (t) is the humidity over a specific period of time t, k 1 is the velocity constant, and k 2 is the capacity constant. The equilibrium moisture content (M e ) can be determined by the Eq. (8).
Eq. (8) together with Eq. (5) (which is used to calculate water loss) will result in Eq. (7) (WL values) which in turn leads to the Eq. (9).
Where k 1 is the intercept in the axis of the ordinates, and k 2 is the parameter of the capacity constant. Likewise, Eq. (8) can be used to calculate solid gain, resulting in the Eq. (10).
Eq. (10) is used to determine weight reduction, resulting in the Eq. (11).
A procedure similar to that performed with Eq. (9) was performed using Eq. (10) and Eq. (11) to calculate values for k 3 , k 4 , k 5 and k 6 , respectively.
2.5. Osmotic process statistical analysis
Statistical data analysis was carried out through a completely randomized design (CRD). All experiments were performed in triplicate. Factors considered in this study include solution type (S1, S2), solution concentration (15% - 5%; 25% - 5%; 35% - 5%) and dehydrating time (40 min, 80 min, 120 min, 160 min). Also, an analysis of variance was performed and proper treatment was chosen based on variables such as the lowest moisture rate obtained from the samples. This was determined using orthogonal polynomials by detecting significant differences between treatments at a 5% significance level (p≤0.05), and modeling the behavior of the response variable. In this way, it was possible to predict the behavior of the response variable using the resulting models.
3. Results and discussions
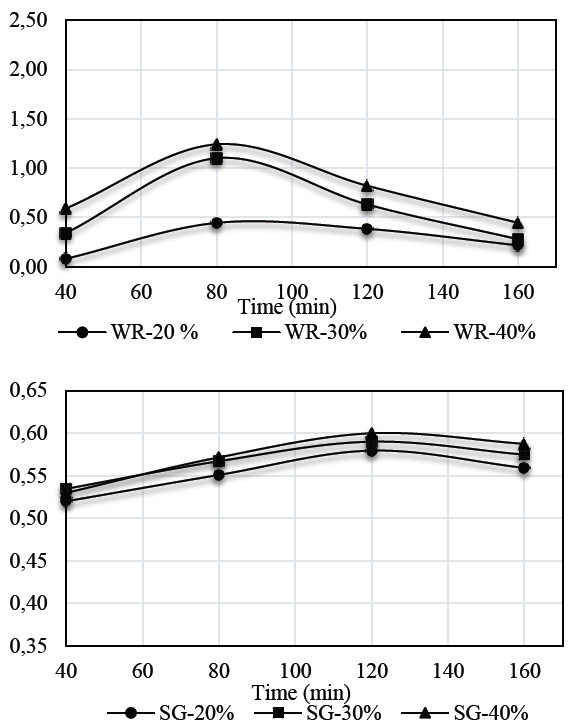
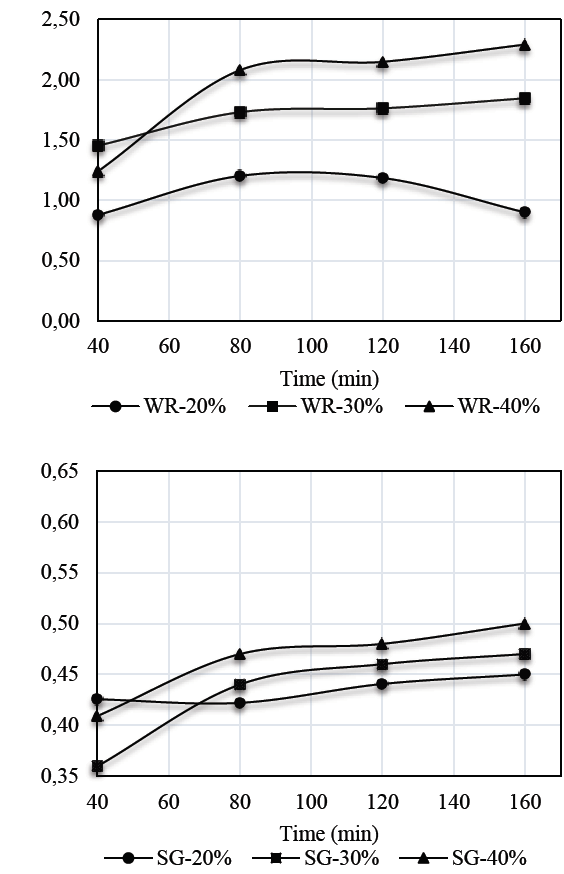
Figs. 2 and 3 show the results for weight reduction (WR) and solid gain (SG) kinetics in auyama samples.
Weight reduction kinetics obtained for S1 solution in samples of auyama, suggests that the highest rate of weight reduction occurred during the initial periods of time as a result of the driving force of the osmotic pressure of the solution over the matrix, thus affecting mass exchange to a greater extent. Over time, the samples experienced a decrease in weight reduction and became more stable, indicating that the osmotic equilibrium occurred during the upper levels of the process [14]. It is important to note that a direct relationship between solution concentration and weight reduction capacity occurred during the osmotic process. Therefore, an increase in concentration levels will result in more significant weight reduction rates, which promotes mass transfer. This means that the higher concentration levels in the solution, the lower the weight reduction values. This is consistent with the results reported for S2 and with those reported by [15,16], who claim that the higher the concentration of the solution or the temperature, the higher the rate of mass transference to promote product dehydration [17].
Regarding solid gain by using the solutions evaluated, the results showed that there is a direct relationship between solution concentration and the ability to boost solid gain during an osmotic process. Also, as mentioned above, it is in the first minutes of the process where the highest mass transfer rate occurs. Results also showed greater presence of solids on the external surface of the sample, and the concentration at 40% performed better when using solution S1 rather than S2. This may be due to a significant difference between the molecular weights of the sweeteners used, where the molecular weight of the sweetener (Stevia) exceeds 4.46 times the molecular weight of glucose. Therefore, this is a significant advantage regarding the effectiveness of both the dehydration process and solid gain [18,19].
Table 1 shows the results reported for the diffusion coefficients of the osmotic process and the constants resulting from Peleg's equation for each of the parameters.
Table 1 Diffusion coefficients and Peleg constants obtained experimentally
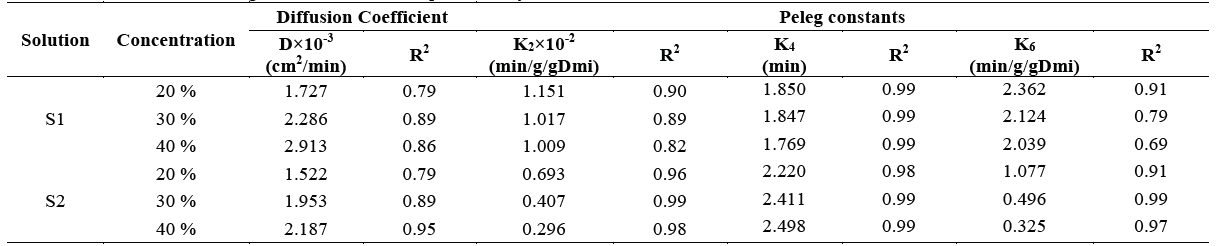
Where K2 is the capacity constant for weight loss (WL), K4 is the capacity constant for solid gain (SG), and k6 is the capacity constant for weight reduction (WR).
Source: The Authors.
The above results suggest that the use of solution S1 results in higher diffusion coefficient values with respect to the solution S2 in all the concentrations. This means that the solution composed of stevia, salt and water offers less resistance to mass transfer when higher concentrations of hypertonic solutions are used. Therefore, S1 performs better than S2 in this regard which accounts for the results obtained with respect to kinetics of weight reduction and solid gain. The above is consistent with that mentioned by Phisut, Azoubel and Murr [20,21], who affirm that the difference in osmotic potential between the solution and the sample will result in a higher rate of solute and water diffusion. Results similar to those reported for the diffusion coefficient were obtained in samples of dehydrated starfruit and West Indian cherries, where the higher the concentration, the higher the diffusion coefficient.
This confirms the direct relationship between the solution concentration and resistance to mass transfer [22,23].
Table 2 shows the results obtained from the analysis of variance, where p-value was less than the significance level (p≤0.05) for the solution-concentration-time interaction. Therefore, there is sufficient statistical evidence to state that the variability in the rate of humidity for each treatment is not influenced by interaction between the factors.
Table 2 Analysis of variance for osmotic dehydration.
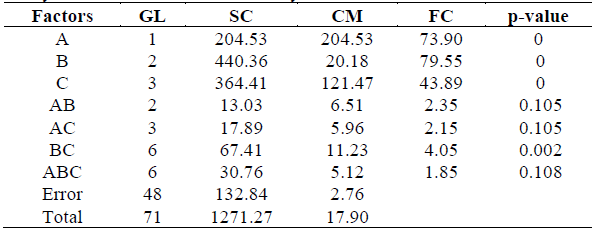
A= Solution, B=Concentration, C= Time
Source: The Authors.
Regarding 2-way interactions a p-value less than the level of significance was obtained for the BC (concentration-time) interaction only, which indicates that there is sufficient statistical evidence to affirm that this interaction is quite significant. Therefore, significant differences were detected for the mean values of the moisture content in osmotically dehydrated pumpkin due to interaction between concentration and time. Based on this result, polynomial adjustment was carried out for the remaining combinations as shown in Table 3.
Table 3 Analysis of time in relation to concentration.
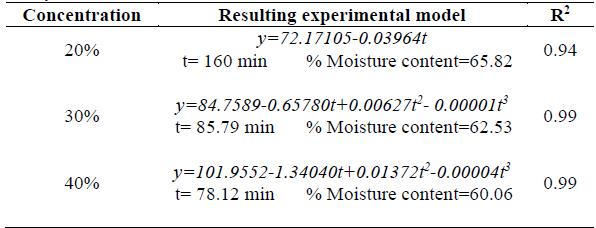
Where y stands for moisture rate.
Source: The Authors.
With regard to time-concentration interaction it should be mentioned that the lowest moisture content rate reported for dehydration process was 60.06 % by using a 40 % w/w concentration over a 78.12-min period. Therefore, it can be deduced that lower moisture rates can be obtained by using intermediate dehydrating times. This phenomenon can be attributed to the fact that mass exchange occurs more quickly during the first stages of the osmotic dehydration process, until equilibrium is reached [24,25]. Similar results were reported for osmotic dehydration of yacon using a sucrose-water solution, where the greatest water loss rate occurred during the first 60 minutes, and no significant changes in water content of the resulting product were observed after this period [11].
Solutions played significant role when the moisture content of auyama samples was analyzed independently. Table 4 shows the results of Tukey's range test which was used to find the ternary combination that best fits osmotic dehydration of auyama samples.
Tabla 4 Tukey's range test for solutions.

Different letters indicate significant statistical differences (p≤0.05).
Source: The Authors.
The above results suggest that the stevia-salt-water solution has the lowest moisture rate in tests with osmotically dehydrated pumpkin, which is statistically significant regarding the value reported for the glucose-salt-water solution. Therefore, it can be deduced that the latter solution performed better when it comes to osmotic dehydration of auyama samples.
4. Conclusions
The use of ternary solutions in osmotic dehydration of auyama samples results in higher mass transfer rates that occur in the first 120 min of the process, in all the concentrations evaluated. In addition, the use of a stevia-salt-water solution leads to greater weight reduction and solid gain rates than those of the glucose-salt-water solution in terms of concentration. Likewise, the diffusion coefficient values obtained for the stevia-salt-water solution show that the higher the concentration of the hypertonic solution, the lower the resistance to mass transfer in auyama samples. Last but not least, osmotic dehydration by using a stevia-salt-water solution performed better than the glucose-salt-water solution in terms of efficiency. This can be considered as a healthy alternative to conventional dehydrated products, especially regarding consumption of high-calorie sweeteners.