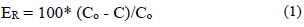1. Introduction
Mining has increased its operation in the last years in Colombia, especially gold mining, which uses highly toxic compounds such as sodium cyanide and mercury as main supplies to recover the metal.
To recover gold small miners use small mining plants or traditional places (known as entables ) where sodium cyanide is used (process known as cyanidation) and in some cases mercury (process known as amalgamation) to process the mineral that contains gold and so get it in pure form or accompanied by silver. During this process solution with high contents of cyanide and heavy metals are generate; most of these plants dispose their wastes in water sources without a previous treatment or with a deficient one, without following the permissible limits for disposals of these compounds in open water.
During the cyanidation process, the residual solutions are charged of metals (lead, iron, copper, zinc among others) that are present in the mineral since they are dissolved by the cyanide during the process. It is most likely the waste of these processes have high contents of cyanide and heavy metals that must be treated before being dumped in open waters, to avoid poisoning, health problems and irreparable damages in the environment such as loss of biodiversity.
A typical effluent of the cyanidation process in a process plant may content cyanide concentrations between 500 y 5500 mgCN/L [1], compared to the permissible limit for mining dumping which is 1 mgCN/L (Resolution No. 0631 March 17th 2015 Environmental and Sustainable Development Ministry), while for heavy metals, they vary according to the type of mineral that is being processed and their mineralogical composition.
It is difficult to give exact data of the size of this problem, because many gold benefit processes made by the traditional miners are done without any registration or permission and are labeled as illegal miners and is not possible to determine the real amount of pollution impact generated by these types of wastes in the open waters.
Worldwide there are many alternatives to remove cyanide and eliminate heavy metals; some of these alternatives have been applied to the treatment of mining effluents. [2-9]. The cyanide degradation with hydrogen peroxide has the objective to transform it in less toxic substances and more unstable, like cyanates, that in the presence of water, become into carbonates and nitrates [10].
The activated carbon is a denomination applied to a whole series of derivative products of carbonaceous materials; is an amorphous material that presents an exceptionally high surface area, and is characterized by having a high proportion of micropores (pores less than 2 nanometers). These characteristics confer them exceptional adsorbent properties that may be exploited in different applications, like in the treatment of sewage waters and the purification of water for human consumption [11].
The use of good management practices in the treatment and implementation of clean methodologies in gold recovery process, will allow to impact in a positive way not only the environment but also to give choices to the traditional miners to carry out their economic activity without any legal issue, since in some cases traditional miners are labeled as criminals for polluting the environment, this will allow to contribute in the legalization of their processes and keep them according to the environmental rules, besides allowing mining to continue being a recognized economic activity as important for big companies as it is for traditional miners. According to the above, the objective of this investigation was to evaluate the removal of cyanide and heavy metals in liquid effluents of gold benefit, through the adsorption with activated carbon and hydrogen peroxide.
2. Materials and methods
2.1. Location of the experiment and sample collection
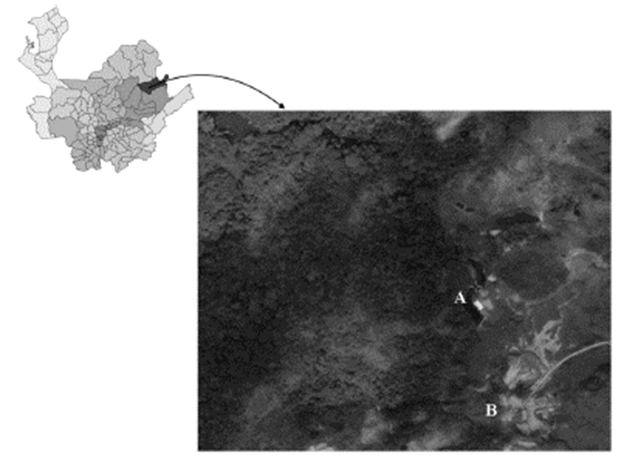
The study took place in the beneficiation plant La Cristalina located in the municipality of Segovia (Antioquia) which belongs to Manzanillo mining society (Fig. 1). Since the flushes are not continuous and they are made once the process has ended, a simple cpound of the effluent residual liquids was taken. The sampling was done during the tank flushing time (2 hours 30 minutes) every 15 minutes, taking 10 samples, 8 liters each; the total sample to run the tests was the compound of the taken samples.
The experimental trials were done in the lab of the plant La Cristalina, as with the cyanide analysis (volumetric analysis Ref. SM 4500-CN); the analysis of the variable response for lead, zinc and iron, took place in the CIMEX lab (Extractive Metallurgic Investigation Center) of the Universidad Nacional de Colombia located Medellín through the atomic adsorption method (air-acetylene flame Ref SM 3111 B).
2.2. Adsorption efficiency process with activated carbon in the removal of cyanide and heavy metals Pb, Zn y Fe
Seven liters of the compound sample, form the effluent under study, were added in a plastic container. The sample was put in contact with activated carbon and it was homogenized through 140 RPM shaking. For the purpose of analyzing the effect of the amount of carbon per liter in the effluent, three dozes of carbon were tested (20, 40 and 60 g/L), taking 40 g of carbon /L of treating solution as mean value, based on similar tests run in the metal chrome (Cr) [9]. It was also evaluated the effect of the time in the percentage of the removal of the elements to analyze. The concentrations of each elements were set out in three different adsorption times (4, 8, 12 hours), taking the 12-hour-lapse as reference, because is the lapse which the elements adsorbed by the carbon have already been stabilized and don’t present any meaningful change; this lapse is based on similar Cr [9] adsorption tests. The tests were done by duplicate (Table 1). In the definition of the base line of the original sample to treat, 3 compound samples were taken to be analyzed. For each sample a check test was done, which was shaken to analyze the variation of the concentrations of the compounds during the time without the presence of activated carbon. In the present trial the concentration variables of cyanide, lead, zinc and iron were analyzed.
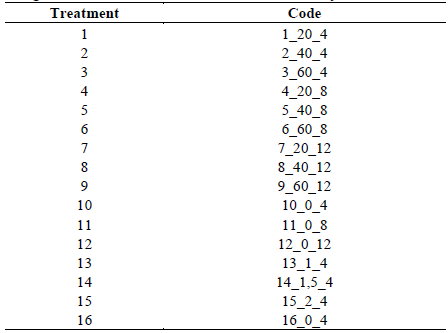
Table 1 Treatments to evaluate in the determination of efficiency of the adsorption with activated carbon in the removal of cyanide and heavy metals.
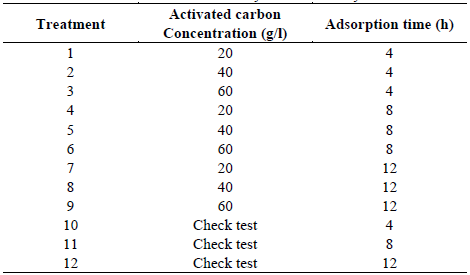
Source: The Authors.
To determine the efficiency of the adsorption of the different metals, with activated carbon, the eq. (1) was used:
Where Co is the initial concentration of the element (CN, Pb, Zn y Fe) and C is the concentration in a determined time (t). The calculation was made in an independent form for every metal.
2.3. Cyanide and heavy metals (Pb, Zn and Fe) degradationprocess efficiency valuation, using hydrogen peroxide,over the previously treated with activated carbon effluent
Start from the previously treated sample, 1 liter was put into a plastic container, to analyze the influence of the peroxide in the effluent. Three different amounts of hydrogen peroxide were tested (1, 1, 5 y 2 L of peroxide/Kg of CN in solution) base on experimental tests done for the degradation of the cyanide [1]. The samples were taken to mechanic shaking for 4 hours, during this time the hydrogen peroxide reacts to degrade and precipitate the heavy metals; once this time has passed the cyanide decomposes in water and oxygen [12]. During the tests the pH was measured every hour to evaluate its variation and at the end of the test the samples were taken to analyze the CN concentration and the determination of metals, these results allowed to evaluate the cyanide degradation process and the precipitation of heavy metals. The tests were performed by duplicate (Table 2).
2.4. Statistical analysis of the information
A variance analysis was done through the R studio program V. 3.5.2, agricolae package version 1.2-8, to determine the occurrence of the meaningful differences between treatments for the group of evaluated variables.
2.5. Design of a methodological proposal for the removal of cyanide and heavy metals in liquid effluents
Once the results have been analyzed a methodological proposal was designed, based on the best results. This proposal relates the implementation of these techniques in a simple form, adapting the conditions to the traditional mining. The proposal consists of a process flowchart with the equipment for the implementation and technical details with the accurate amount of reagent for different amounts of the effluents to be treated and the costs of the treatments
3. Results and discussion
The results obtained were analyzed through non parametric statistics, with the Krustal Wallis model with the R studio program V. 3.5.2, agricolae package version 1.2-8, since the data did not show normality in its distribution. In the presentation of the results an assigned code was used for every treatment, structured and based on the next information: number of treatment_concentration reagent (activated carbon-hydrogen peroxide) time of treatment. The defined codes are shown on the Table 3.
The results obtained for the adsorption of metals and cyanide with activated carbon are shown on the Table 4. In general terms it could be observed that as the amount of carbon increased, the efficiency of the adsorption increased as well as different elements. The pH in the solution did not show any variation by action of the adsorption of the elements in the activated carbon. The biggest percentages were show with 60 grams of activated carbon/L of solution.
Table 4 Concentrations of response variables, after the adsorption treatment with activated carbon.
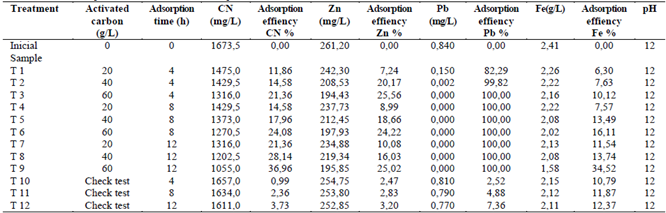
Source: The Authors.
As for the times of adsorption, different behaviors were seen for every element, being lead totally adsorbed in 4 hours with 60 g of carbon / L of solution. Zinc within 4 hours with 60 g of carbon/ L of solution was stabilized and not notorious changes were seen after 8 and 12 hours, which was corroborated through statistical analysis. However, for the cyanide and iron important changes were seen while the adsorption time increased, getting its biggest percentages of efficiency with 60 g of carbon/ L and 12 hours of treatment.
In the Figs. 2 and 3 the statistical analysis is shown. For the understanding of these, the letters on each bar are taking into account, indicating which bars with the same letters do not present meaningful statistic differences.
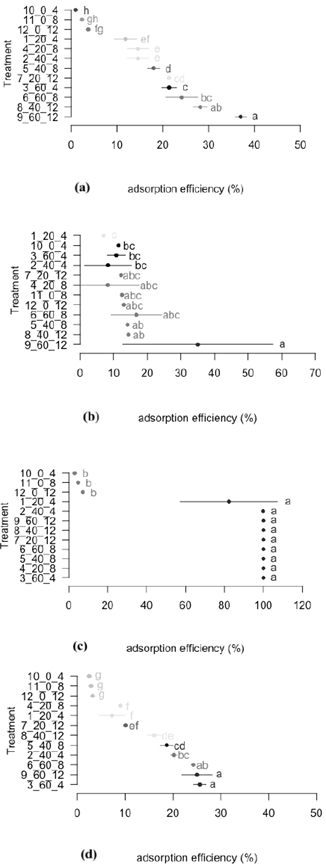
Source: The Authors.
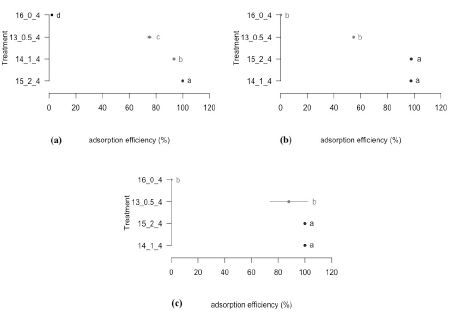
Figure 2 Adsorption efficiency through the treatment with activated carbon. a) Adsorption efficiency for cyanide. b) Adsorption efficiency for zinc. c) Adsorption efficiency for lead. d) Adsorption efficiency for iron. *The same letters don’t have any meaningful difference.
Source: The Authors.
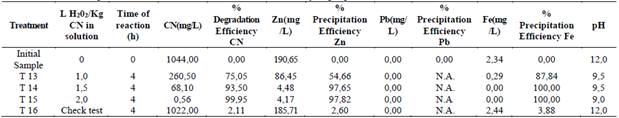
Figure 3 Efficiency of degradation of cyanide and precipitation of lead and iron, through the addition of hydrogen peroxide. a) Cyanide degradation efficiency. b) Zinc precipitation efficiency. c) Iron precipitation efficiency.
In the percentage of cyanide adsorption, using the activated carbon, the best treatments were 8 and 9, which do not have meaningful statistics differences among them (Fig. 2a). Most of the cyanide adsorption efficiency was obtained with the treatment 9 (36,96%), in which a 60 g. activated carbon/L of solution was used for 12 hours. Meaningful statistical differences were observed in the treatments 8 and 9 with the check tests (treatments 10, 11 and 12), confirming the cyanide adsorption with activated carbon when this was added to the solution.
In the Fig. 2b meaningful statistical differences are not observed among the treatments 3, 9 and 6 with relationship to the zinc adsorption efficiency. In these treatments the same amount of activated carbon was used (60 g) in different contact times (4, 12 and 8 hours respectively), noticing that in a lapse of time of over 4 hours there were not meaningful effects in the change of the efficiency. An existing difference was also observed between treatments 3, 9 and the check tests, showing the adsorption effects while the amount of carbon added to the solution increased.
No meaningful statistical differences were shown in the treatments from 1 to 9, in the adsorption of lead with activated carbon, in comparison to the treatments 10, 11 and 12 (check tests) (Fig. 2c). From the treatment 3 to 9, a removal of lead was obtained of 100% showing the effect of the adsorption of activated carbon over lead, in relationship to the check tests, in which meaningful statistical differences were shown, with a maximum of adsorption of 7%.
In the Fig. 2d can be observed that the most removal of iron occurred in the treatment 9 (34,52%) with 60 grams of carbon per litter of solution and 12 hours of contact. This treatment presented meaningful statistical differences with the treatments 1, 2, 3 and 10, in which different amounts of carbon were used, with the same time of contact (4 hours). This indicates that in the efficiency of iron adsorption, there is no influence of the concentration of activated carbon, but the contact time with the effluent to treat.
To select the best treatment in the adsorption of the polluting elements (CN, Zn, Pb and Fe), after applying activated carbon, the results of the statistical analysis were considered (Fig. 2). As selection criteria the one who best behaved in the adsorption of the polluting elements considered in the study was chosen. According to this, it was identified that treatment 9 had this characteristic, because statistically there were meaningful differences with the other treatments, becoming as the best treatment in the adsorption of polluting elements.
The results obtained, allow to notice that the most percentages of adsorption occurred with 60g. of activated carbon per liter of solution, showing a directly proportional behavior between the concentration of carbon and the efficiency of adsorption. When the amount of added carbon was increased in the solution to be treated, the adsorption efficiency of the different polluting elements increases [13]. However in the times of adsorption different behaviors were observed, indicating the need to have a longer lapse of contact time (12 h) when 60 g of carbon/L of solution were used, in the case of cyanide and iron, and in a shorter contact time for the lead and zinc adsorption (4 h), showing different behaviors in the adsorption rate, since for the adsorption to occur, is necessary for the bond between the solute and the solution to be broken [14], in the results, bigger adsorption rate were observed in lead and zinc, compared to cyanide and iron, reflected in the times of adsorption. In the lead adsorption removal efficiencies were obtained between 82,29 and 100% in a 4-hour lapse, which confirms the adsorbent power of the activated coal over this element [9].
The pH of the solution didn´t vary during the adsorption tests, remaining in 12, this is because of the adsorption phenomenon presented in the tests is of the physical type indicating that the bond between activated coal and the removed polluting elements is caused by the Van Der Walls forces, and there was neither chemical reaction nor formation of new compounds, being this a reversible process [14].
The results obtained in the degradation process of the cyanide and precipitation of the metals in addition of the hydrogen peroxide can be seen on the Table 5.
Table 5 Concentration of the response variables, after the treatment with addition of hydrogen peroxide.
Source: The Authors.
The effect of the addition of the hydrogen peroxide in the degradation of cyanide is shown in the Fig. 3a. It is observed that the best efficiency of the cyanide degradation (99, 95%) occurred in the treatment 15 (2 L of hydrogen peroxide/Kg of cyanide in the solution during 4 hours), showing meaningful statistical with the treatments 13 and 14 (efficiency of 75,05 and 93,5%, respectively) and with the check test (treatment 16), which was obtained with a 2,11% efficiency. This indicates that the effect that represents the amount of added peroxide to the solution in the degradation of cyanide.
In the removal of zinc by effect of the precipitation of the hydrogen peroxide, the best efficiencies were obtained with the treatments 14 (97,65%) and 15 (97,82%) (Fig. 3b). Between these two treatments there were not meaningful statistical differences, but there were differences with the check test (treatment 16), with a Zn removal efficiency of 2,6%. This shows the effect of the amount of the added reagent over the efficiency of the zinc removal.
In the Fig. 3c, are observed the results of the removal of iron, which had a similar behavior to the zinc removal. The treatments 14 and 15 do not present meaningful statistical differences among themselves (removal of 100%), but it did with the treatment 13 (87,84%) and with the check test (3,88%). A marked difference was shown in the efficiency when the peroxide was added, in comparison to the treatments in which this one wasn’t added.
To select the best treatment in the hydrogen peroxide addition stage, the graphics presented in the Fig. 3. were analyzed altogether. For the three polluting elements the treatment 15 showed the best adsorption efficiency. Although treatment 14 is a good choice related to the least amount of reagent used, that could decrease the costs of treatment, it was noticed that this one works efficiently for zinc and iron without marked differences in relationship to the treatment 15, however, in the case of cyanide, the treatment 14 is not the best choice. In the case of lead the statistical analysis was not done, since this was totally removed in the activated carbon stage.
In general terms can be observed that as the amount of added hydrogen peroxide in the solution increases, it also increases the efficiency of the degradation of cyanide and the precipitation of metals [1]. In the pH was observed that as the amount of hydrogen peroxide increases this one decreases, since the peroxide is an oxidant agent and after 4 hours it decomposes in water and oxygen. Besides during the change of pH, the precipitation of heavy metal happens in form of hydroxide [12].
The statistical differences observed in the treatments with addition of hydrogen peroxide regarding the check tests, show a trend in the increasing of the efficiency when the reactive is added. The low efficiencies obtained in the check test show that there is a removal low percentage in these samples, that can be attributed to the effect of the added oxygen to the solution by the shaking effect and the natural degradation or cyanide volatilization that can be present in the solution [7]. According to this, the values of the check tests were subtracted from the results obtained, in order to correct this effect noticed in the experiments.
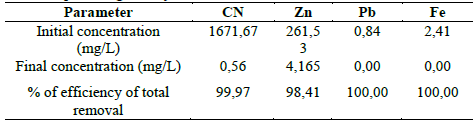
In the Table 6, the results obtained from the combination of the two processes: adsorption with activated carbon and degradation of cyanide and precipitation of heavy metals with hydrogen peroxide. It can be noticed that the initial concentration of the untreated sample and the final concentration of the sample after having been treated with activated carbon and hydrogen peroxide. These dates are projected from the combination of treatments 9 and 15.
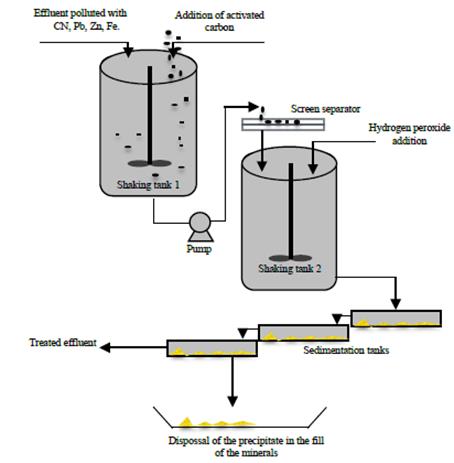
In the Fig. 4 the proposed diagram is observed, for the implementation of the studied removal alternatives in this article. This diagram is based on a methodology of easy application, accessible equipment’s and easy to use for traditional miners.
Source: The Authors.
Figure 4 Flowchart of the cyanide removal process and heavy metals in liquid effluents of traditional mining.
The liquid effluent that contains high levels of cyanide and heavy metals, is put in contact with the activated carbon in a tank and mixed through a shaker (approximately 140 RPM) this process takes 12 hours. After that the mix of the solution and carbon is sent to a second tank, where in the upper part a net or screen separator is set to remove the activated carbon the one has already been impregnated by the elements in the first stage of adsorption. The carbon-free liquid is poured in a second shaking tank where the hydrogen peroxide is added and is left to be shaken during 4 hours, in this second stage some white precipitate are formed by the metallic hydroxides, the mix of the liquid and precipitate is sent to some sedimentation tanks where is separated the precipitate liquid. Once the metallic hydroxides (precipitate) have been removed the solution is treated with low contents of cyanide and metals, and can be dumped. The removed precipitates in the solution are disposed in the landfill sites, plants have to dispose solid residues of the mineral.
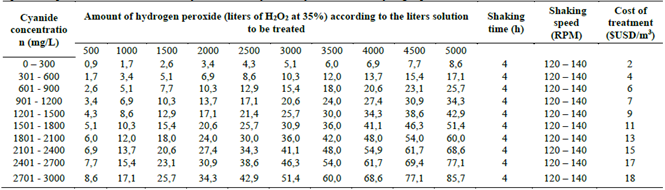
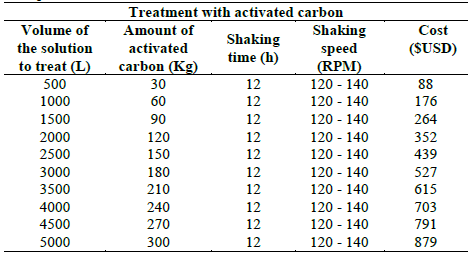
In the Table 7 the operational conditions are represented, cost of the treatment and the amount of carbon for the adsorption stage, depending on the amount of solution to treat.
Table 7 Operational parameters for the removal of cyanide and heavy metals through adsorption with activated carbon, with the cost of treatment.
Source: The Authors.
6. Conclusions
In the adsorption process with activated carbon the best results were obtained with 60 g of activated carbon/L of solution, confirming that as the relationship solid/liquid increases the amount of adsorbed metal increases too [13] getting a 36,89% adsorption efficiency for cyanide, 25,66% for zinc, 34,52% for iron and 100% for lead. This last result confirms the great efficiency of activated carbon in the removal of lead [9]. While the adsorption time for the cyanide and iron was 12 hours, zinc and lead stabilized in a 4- hour lapse; from this lapse they didn’t show any meaningful change.
In the addition process with the hydrogen peroxide to the previously treated sample with activated carbon, the best results were obtained in the treatment 15 (2 L de H2O2/Kg de CN in solution), getting a cyanide degradation efficiency of 99,95% and a precipitation of zinc of 97,82% and iron of 100% , confirming the efficiency of the hydrogen peroxide in the cyanide degradation [1] and the theory of the capacity of hydrogen peroxide to precipitate metals and this way to be removed from the solution [12]. No results were shown for lead since it was totally eliminated in the adsorption with activated carbon stage and the sample to treat with peroxide had no longer lead.
With the combination of the adsorption process with activated carbon and after a treatment with hydrogen peroxide it was possible to get to a concentration of cyanide of 1.671 to 0,56 mg/L, zinc of 261,53 to 4,165 mg/L, lead of 0,84 to 0 mg/L, iron of 2,41 to 0 mg/L, getting to decrease in a meaningful way the concentrations up to permissible limits for cyanide, lead, iron and approaching to the permissible limits for the concentration of zinc (Ruling No. 0631 of 2005) (Permissible limits in mining dumps: cyanide: 1 mg/L, lead: 0,2 mg/L, zinc: 3 mg/L, iron: 2 mg/L).
The great statistical differences that were seen in the treatments with addition of activated coal and hydrogen peroxide regarding the samples without addition of any decontaminating agent, only with shaking.
(Check tests), show the effect in the efficiency of the removal when the treatment methodology proposals are implemented, in contrast with the low efficiencies obtained in the check tests, although the values of these treatments are low, they show the effect that shaking produces, therefore the addition of the oxygen in the solution, phenomenon that causes one of the methodologies known as natural degradation or cyanide volatilization [7]. However, these natural processes are low-efficient requiring days even months to degrade the compounds, besides in the case of the heavy metals don’t degrade, they bio cumulate, confirming the need to implement efficient alternatives in these type of effluents before being offloaded in open waters in order to avoid irreversible damages in the ecosystem.
Regarding the costs of the treatment, the adsorption process represents most of the part of the total cost (90-98%), in comparison to the cost of the treatment with hydrogen peroxide 2-10% of the total cost. Although in this research the two treatment methods (activated carbon and hydrogen peroxide) are not compared as individual alternatives, but as complementary methods that by consecutive combination of these allow to get to permissible levels, if we can say that the hydrogen peroxide is an alternative beyond efficient economical to treat cyanide and heavy metals.