1. Introduction
Aspergillus niger is used worldwide in the industrial production of citric acid by submerged fermentation [1-7]. However, under specific factors, the A. niger accumulate oxalic acid as a by-product [7] and, organic acid production can be stimulated to result in a quantitative conversion of carbon substrate into acids [8]. The application of several methods for citric and oxalic acids determination, e.g., enzymatic methods, gas chromatography and liquid chromatography, allow simultaneous analyses of organic acids [9].
Although ion exclusion chromatography is usually considered unsatisfactory for acid determination because of the poor peak resolution [10], in this work the sample preparation improve the analysis, due to the reduction of the interferences with non-consume reagents from culture medium or metabolites produced in the fermentation process that can affect the quantification and identification of acids. Furthermore, the fact of the oxalic acid co-elutes in the dead volume [11].
Solid-phase extraction (SPE) is a useful and functional sample preparation method [12,13], with improvements in the quantification after using it, in comparison with samples that did not use purification systems. Method selectivity on the determination of citric and oxalic acid in the fermentation broth is essential to understand the substrate-product relationship and carry out detailed monitoring and control that allow the design of specific culture medium and, optimize operation times to increase the metabolite production rate [14].
The purpose of this investigation was to develop a method that includes sample preparation and HPLC analysis, allowing the determination and quantification of organic acids from a fermentation broth, being selective for citric and oxalic acid.
2. Materials and methods
2.1. Reagents
Sulfuric acid (98% analytical grade), methanol (99.96% analytical grade), citric and oxalic acid HPLC standards were provided by Merck (USA). The mobile phase and sample preparation used deionized water, obtained from a Synergy® water purification system.
2.2. HPLC system and method validation
The quantification of citric and oxalic acid was carried out in an HPLC Prominence (Shimadzu Corporation, USA) controlled by a workstation with Lab Solutions software and, equipped with a UV/VIS diode array detector- DAD at 210 nm [15].
IC-Pak Ion-Exclusion 7µm (7.8 x 300 mm) (Waters, USA) column at 40°C was used for HPLC analyses with an isocratic mode at a flow rate of 0.6 mL/min and an injection volume of 10 µL. For method optimization, mobile phase (Sulfuric acid) was tested at concentrations ranging from 0.001 to 0.1N.
A serial dilution of citric and oxalic acid was used for the construction of the calibration curve. The filtered standards were analyzed in triplicates for calibration, using an HPLC System [16]. The validation parameters consider linearity range, precision, accuracy, detection and quantification limits [17]. The peaks were identified by their retention times, comparing the UV-visible spectra and spiking with standards [18]. Precision and accuracy were determined using the mean of consecutive injections of standard mixtures of citric and oxalic acid at different concentration levels, considering the relative standard deviation (%RSD). The analyses were carried out in two separate sessions (7 days), using the same method, analyst, and equipment.
2.3. Fermentation
Aspergillus niger spores were inoculated in 100 mL of culture medium containing 140 g/L of Sucrose, 1.5g/L of KH2PO4, 1.5g/L of K2HPO4, 0.8 g/L of MgSO4.7H2O, 4 g/L of (NH4)2SO4, 0.5 g/L of ZnSO4.7H2O and 0.5 g/L of Fe2(SO4)3. The initial pH of the culture medium was adjusted and autoclaved at 110°C for 10 minutes to avoid Maillard reaction. The fermentation was carried out in 500 mL Erlenmeyer flasks with orbital agitation at 180 rpm, 30°C for 9 days.
2.4. Sample preparation
Centrifugation of the fermented broth was used to separate aqueous phase from biomass and supernatant filtered through 0.2 μm PTFE filter [19]. To archive, the best resolution and precision, direct injection and treated sample using SPE-Strata Sax cartridges (Phenomenex) were compared, with the purpose of separating neutral compounds (e.g., sugars and other metabolites) from the acidic ones [20]. A cartridge was conditioned and, the sample (1 mL) adjusted to pH 9, loaded through the cartridge ensuring a flow rate of 0.3 mL/min. The interferences were washed out using 1 mL of deionized water, while acid compounds were eluted five times with 0.5 mL of HCl (1 N), diluted with the mobile phase to a final volume of 5 mL, filtered through 0.2 µm syringe filter and later injected on HPLC [21,22].
3. Results and discussion
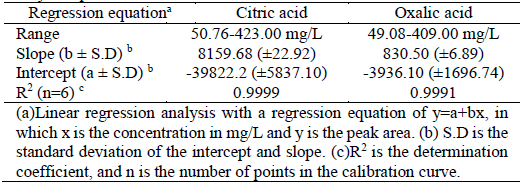
Preliminary test with fermentation broth established a calibration range between 50-400 mg/L of citric and oxalic acid mixtures. The concentration peak area relationships were described by simple regression analysis [23,24]. Calibration curve exhibited good linearity (R2> 0.999) and the regression parameters are listed in Table 1. According to Monteiro Coelho et al. [25], the R-value for the calibration curves must be higher than 0.99, which verifies that the linearity achieved in this study for the response to external standards is adequate for this purpose.
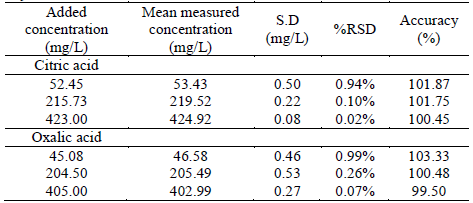
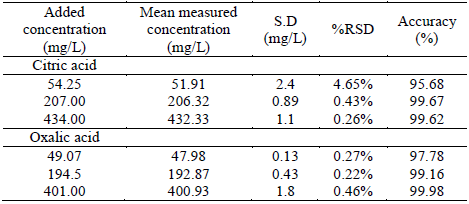
Three control samples into the calibration range were used to determine the accuracy and precision (Tables 2 and 3). Results of concentration were used to verify the procedure repeatability and system operation regarding stability and precision [26]. The acceptance criteria for %RSD of concentration in method validation is ≤ 5% [21] and includes possible variations in injection, flow, and others. Results were similar to literature and indicated that the method was precise and highly reproducible for target compounds [15].
Table 2 Accuracy and precision tests performed with three samples on the calibration day
Source: The Authors.
Table 3 Accuracy and precision tests performed with three samples a week after calibration.
Source: The Authors.
Accuracy values are within the established criteria (±10%) [21,27]. LOQ values of 50.76 mg/L (%RSD=1.64) to citric acid and 20.18 mg/L (%RSD=0.65) to oxalic acid, indicating method sensibility. LOD for both analytes were 0.6 mg/L. For future method applicability, these values are adequate since the concentration or citric and oxalic acids exceed these amounts in the samples analyzed.
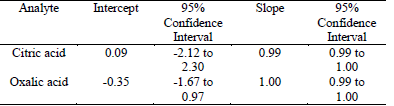
The linear regression of the recovery curve (analyte concentration quantified by the method against the nominal concentration) was used to determine the systematic error. Table 4 shown the confidence interval of the intercept and the slope was calculated to p=0.05 [28] for every recovery function having either: an intercept > 0 and slope < 1 (citric acid) or an intercept < 0 and slope > 1 (oxalic acid), indicating no presence of systematic error during method development and the validation process.
Table 4 Linear regression values to recovery curve, the concentration of the analyte quantified by the method against the nominal concentration
Source: The Authors.
In a preliminary phase, we tested different concentrations of sulfuric acid as the mobile phase to obtain the best chromatographic conditions for citric and oxalic acid determination and retention time was slightly influenced by the changing concentration of the eluent (data not shown). A concentration of 0.001 N achieved an accurate separation with acceptable baseline stability and small background noise and using these conditions, the separation of target compounds is less than 10 minutes. According to Wang et al. [29], the column temperature affects acid determination: as the column temperature increase, the retention time of the organic acids decreased, but weakly. Thus, a column temperature of 40°C was selected to develop the HPLC method.
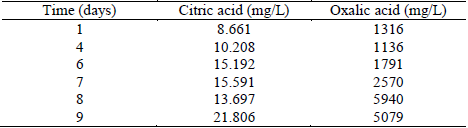
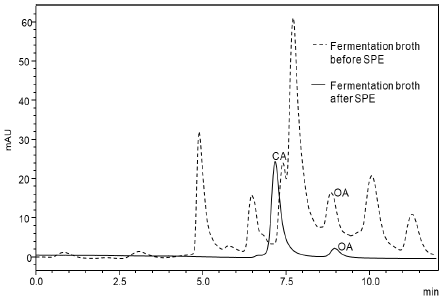
The method was applied to determine the sample composition at different times during the fermentation process. The results are presented in Table 5, and Fig. 1 shows that citric and oxalic acid could be detected during the fermentation process and, the production of citric acid was lower compared with oxalic acid due to the presence of factors that affect the citric acid production, such as the type and concentration of the carbon and nitrogen source, the limitation of phosphate and the pH of the culture medium. Glucose does not absorb UV wavelengths and not interfere with the detection of organic acids. However, fructose and other metabolites produced by Aspergillus niger (e.g., proteins, oligosaccharides) show UV absorption [30,31], hindering organic acids quantification (Fig. 1).
Table 5 Concentration change of citric and oxalic acids during the fermentation process.
Source: The Authors.
Source: The Authors.
Figure 1 Representative chromatogram of fermentation broth sample after and before the SPE treatment. For chromatography, conditions refer to HPLC system and method validation section.
The sample treatment was necessary to cut interferences of some compounds that could affect the analysis results. The SPE chosen for this study was ion-exchange, the quaternary amino group in this cartridge remains positively charged under all conditions, offering a strong ion-exchange retention mechanism [32]. This type of cartridge is used for isolation of acidic compounds, including the carboxylic acids. The matrix was an aqueous organic sample with salts concentration < 0.1M and pH of the sample should be 2 pH units higher than the highest pKa of the organic acids of interest to make sure that all acids are deprotonated. Elution and washing rate were necessary to allow ideal retention through the cartridge to avoid loss of analyte and promote interaction between mobile and stationary phase [33]. Volumes lower than 2.5 mL did not allow the complete recuperation of the acids, and higher than 2.5 mL gave the same recovery results but diluted the sample unnecessarily [34].
The sample preparation had effects on the retention time of analytes in the column used: a pH decrease causes a retention increase of weakly ionized analytes as carboxylic acids, due to a reduction of the ionized analytes fraction (Fig. 1). Small variations in retention times (± 0.1 minutes) are acceptable for method development. However, the historical behavior of the method should be used to decide what is adequate with large biological molecules [35]. If the sample has many peaks, is hard to separate the target compounds, especially if there are minor differences in retention times. Factors like the gradual accumulation of impurities in the column can affect the analysis, modifying the separation efficiency and the increase in the backpressure of the mobile phase.
The results confirm when the method ensures that all quality requirements are fulfilled. Complete validation for HPLC system is very time-consuming and involves many different steps during sample preparation, thus are increasing the number of possible sources of error. For this reason, a validation procedure with few parameters to control a considerable number of variables is suitable [36]. In this study, a simple calibration procedure evaluated retention times, peaks and method specificity.
4. Conclusions
The SPE-HPLC-DAD method described in this work allows the monitoring of citric and oxalic acids in the fermentation process. The sample preparation with SPE for carboxylic acids extraction from fungi fermentation broth using the ion-exclusion technology is probably one of the most common applications of sample purification and provides an adequate alternative that excludes interferences and supports a properly quantification and better chromatogram definition. A simple method with acceptable precision, accuracy and linearity have been development and validation for simultaneous determination of citric and oxalic acids in fungi fermentation broth. Finally, this method is useful for analyzing citric, and oxalic acid in Aspergillus niger fermentation broth and can be applied in the analysis of other fungi fermentation broth.